Temozolomide in the treatment of high-grade gliomas in children: a report from the Children's Oncology Group
- PMID: 21339192
- PMCID: PMC3064602
- DOI: 10.1093/neuonc/noq191
Temozolomide in the treatment of high-grade gliomas in children: a report from the Children's Oncology Group
Abstract
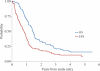
To determine whether temozolomide is an active agent in the treatment of children with high-grade astrocytomas and whether survival is influenced by the expression of the O6-methylguanine-methyltransferase gene (MGMT) in these patients. In the Children's Oncology Group study ACNS0126, 107 patients with a diagnosis of anaplastic astrocytoma (AA), glioblastoma multiforme (GBM), or gliosarcoma were enrolled. All patients underwent concomitant chemoradiotherapy with temozolomide, followed by adjuvant chemotherapy with temozolomide. The outcomes were compared with those of children treated in Children's Cancer Group (CCG) study CCG-945. Formalin-fixed, paraffin-embedded tumor tissue was available in 71 cases for immunohistochemical analysis of MGMT expression. Ninety patients were deemed eligible, 31 with AA, 55 with GBM, and 4 with other eligible diagnoses. The 3-year event-free survival (EFS) and overall survival (OS) rates were 11 ± 3% and 22 ± 5%, respectively. There was no evidence that temozolomide given during radiation therapy and as adjuvant therapy resulted in improved EFS compared with that found in CCG-945 (p = 0.98). The 3-year EFS rate for AA was 13 ± 6% in ACNS0126 compared with 22 ± 5.5% in CCG-945 (p = 0.95). The 3-year EFS rate for GBM was 7 ± 4% in ACNS0126 compared with 15 ± 5% in CCG-945 (p = 0.77). The 2-year EFS rate was 17 ± 5% among patients without MGMT overexpression and 5 ± 4% among those with MGMT overexpression (p = 0.045). Temozolomide failed to improve outcome in children with high-grade astrocytomas. MGMT overexpression was adversely associated with survival.
Figures
Comment in
-
Temozolomide for pediatric high-grade gliomas.Curr Neurol Neurosci Rep. 2012 Apr;12(2):111-3. doi: 10.1007/s11910-012-0250-2. Curr Neurol Neurosci Rep. 2012. PMID: 22249491 No abstract available.
References
-
- Finlay JL, Boyett JM, Yates AJ, et al. Randomized phase III trial in childhood high-grade astrocytoma comparing vincristine, lomustine, and prednisone with the eight-drugs-in-1-day regimen. Children's Cancer Group. J Clin Oncol. 1995;13:112–123. - PubMed
-
- Boyett J, Yates A, Gilles F, et al. When is a high-grade astrocytoma (HGA) not a HGA? Results of a central review of 226 cases of anaplastic astrocytoma (AA), glioblastoma multiforme (GBM), and other-HGA (OTH-HGA) by five neuropathologists. Proc Am Soc Clin Oncol. 1998;17:526a.
-
- Athanassiou H, Synodinou M, Maragoudakis E, et al. Randomized phase II study of temozolomide and radiotherapy compared with radiotherapy alone in newly diagnosed glioblastoma multiforme. J Clin Oncol. 2005;23:2372–2377. - PubMed
-
- Jalali R, Basu A, Gupta T, et al. Encouraging experience of concomitant temozolomide with radiotherapy followed by adjuvant temozolomide in newly diagnosed glioblastoma multiforme: single institution experience. Br J Neurosurg. 2007;21:583–587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials