Investigating the early stages of photosystem II assembly in Synechocystis sp. PCC 6803: isolation of CP47 and CP43 complexes
- PMID: 21339295
- PMCID: PMC3083219
- DOI: 10.1074/jbc.M110.207944
Investigating the early stages of photosystem II assembly in Synechocystis sp. PCC 6803: isolation of CP47 and CP43 complexes
Abstract
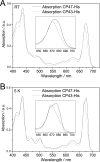
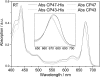
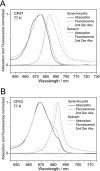
Biochemical characterization of intermediates involved in the assembly of the oxygen-evolving Photosystem II (PSII) complex is hampered by their low abundance in the membrane. Using the cyanobacterium Synechocystis sp. PCC 6803, we describe here the isolation of the CP47 and CP43 subunits, which, during biogenesis, attach to a reaction center assembly complex containing D1, D2, and cytochrome b(559), with CP47 binding first. Our experimental approach involved a combination of His tagging, the use of a D1 deletion mutant that blocks PSII assembly at an early stage, and, in the case of CP47, the additional inactivation of the FtsH2 protease involved in degrading unassembled PSII proteins. Absorption spectroscopy and pigment analyses revealed that both CP47-His and CP43-His bind chlorophyll a and β-carotene. A comparison of the low temperature absorption and fluorescence spectra in the Q(Y) region for CP47-His and CP43-His with those for CP47 and CP43 isolated by fragmentation of spinach PSII core complexes confirmed that the spectroscopic properties are similar but not identical. The measured fluorescence quantum yield was generally lower for the proteins isolated from Synechocystis sp. PCC 6803, and a 1-3-nm blue shift and a 2-nm red shift of the 77 K emission maximum could be observed for CP47-His and CP43-His, respectively. Immunoblotting and mass spectrometry revealed the co-purification of PsbH, PsbL, and PsbT with CP47-His and of PsbK and Psb30/Ycf12 with CP43-His. Overall, our data support the view that CP47 and CP43 form preassembled pigment-protein complexes in vivo before their incorporation into the PSII complex.
Figures

References
-
- Wydrzynski T. J., Satoh K. (eds) (2005) in Photosystem II: The Light-driven Water:Plastoquinone Oxidoreductase, Springer, Dordrecht, The Netherlands
-
- Zouni A., Witt H. T., Kern J., Fromme P., Krauss N., Saenger W., Orth P. (2001) Nature 409, 739–743 - PubMed
-
- Ferreira K. N., Iverson T. M., Maghlaoui K., Barber J., Iwata S. (2004) Science 303, 1831–1838 - PubMed
-
- Guskov A., Kern J., Gabdulkhakov A., Broser M., Zouni A., Saenger W. (2009) Nat. Struct. Mol. Biol. 16, 334–342 - PubMed
-
- Loll B., Kern J., Saenger W., Zouni A., Biesiadka J. (2005) Nature 438, 1040–1044 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

