Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation
- PMID: 21339324
- PMCID: PMC3058586
- DOI: 10.1084/jem.20102131
Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation
Abstract
The human breast tumor microenvironment can display features of T helper type 2 (Th2) inflammation, and Th2 inflammation can promote tumor development. However, the molecular and cellular mechanisms contributing to Th2 inflammation in breast tumors remain unclear. Here, we show that human breast cancer cells produce thymic stromal lymphopoietin (TSLP). Breast tumor supernatants, in a TSLP-dependent manner, induce expression of OX40L on dendritic cells (DCs). OX40L(+) DCs are found in primary breast tumor infiltrates. OX40L(+) DCs drive development of inflammatory Th2 cells producing interleukin-13 and tumor necrosis factor in vitro. Antibodies neutralizing TSLP or OX40L inhibit breast tumor growth and interleukin-13 production in a xenograft model. Thus, breast cancer cell-derived TSLP contributes to the inflammatory Th2 microenvironment conducive to breast tumor development by inducing OX40L expression on DCs.
Figures

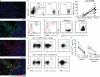
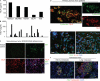
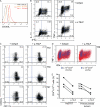


Comment in
-
Immunotherapy: TSLP fuels inflammation in breast and pancreatic tumors.Nat Rev Clin Oncol. 2011 May;8(5):254. doi: 10.1038/nrclinonc.2011.40. Nat Rev Clin Oncol. 2011. PMID: 21451464 No abstract available.
-
Tumour immunology: TSLP drives human tumour progression.Nat Rev Immunol. 2011 Apr;11(4):235. doi: 10.1038/nri2961. Nat Rev Immunol. 2011. PMID: 21553711 No abstract available.
-
TSLP1: a new piece in the puzzle of tumor-associated Th2-type inflammation.Immunotherapy. 2011 Jun;3(6):713-7. doi: 10.2217/imt.11.67. Immunotherapy. 2011. PMID: 21668306 No abstract available.
References
-
- Aspord C., Pedroza-Gonzalez A., Gallegos M., Tindle S., Burton E.C., Su D., Marches F., Banchereau J., Palucka A.K. 2007. Breast cancer instructs dendritic cells to prime interleukin 13–secreting CD4+ T cells that facilitate tumor development. J. Exp. Med. 204:1037–1047 10.1084/jem.20061120 - DOI - PMC - PubMed
-
- Bell D., Chomarat P., Broyles D., Netto G., Harb G.M., Lebecque S., Valladeau J., Davoust J., Palucka K.A., Banchereau J. 1999. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J. Exp. Med. 190:1417–1426 10.1084/jem.190.10.1417 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

