Disrupted junctional membrane complexes and hyperactive ryanodine receptors after acute junctophilin knockdown in mice
- PMID: 21339484
- PMCID: PMC3056402
- DOI: 10.1161/CIRCULATIONAHA.110.006437
Disrupted junctional membrane complexes and hyperactive ryanodine receptors after acute junctophilin knockdown in mice
Abstract
Background: Excitation-contraction coupling in striated muscle requires proper communication of plasmalemmal voltage-activated Ca2+ channels and Ca2+ release channels on sarcoplasmic reticulum within junctional membrane complexes. Although previous studies revealed a loss of junctional membrane complexes and embryonic lethality in germ-line junctophilin-2 (JPH2) knockout mice, it has remained unclear whether JPH2 plays an essential role in junctional membrane complex formation and the Ca(2+)-induced Ca(2+) release process in the heart. Our recent work demonstrated loss-of-function mutations in JPH2 in patients with hypertrophic cardiomyopathy.
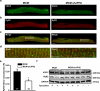
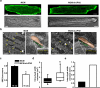
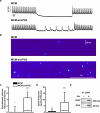
Methods and results: To elucidate the role of JPH2 in the heart, we developed a novel approach to conditionally reduce JPH2 protein levels using RNA interference. Cardiac-specific JPH2 knockdown resulted in impaired cardiac contractility, which caused heart failure and increased mortality. JPH2 deficiency resulted in loss of excitation-contraction coupling gain, precipitated by a reduction in the number of junctional membrane complexes and increased variability in the plasmalemma-sarcoplasmic reticulum distance.
Conclusions: Loss of JPH2 had profound effects on Ca2+ release channel inactivation, suggesting a novel functional role for JPH2 in regulating intracellular Ca2+ release channels in cardiac myocytes. Thus, our novel approach of cardiac-specific short hairpin RNA-mediated knockdown of junctophilin-2 has uncovered a critical role for junctophilin in intracellular Ca2+ release in the heart.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

