Kaposi sarcoma herpesvirus (KSHV) vFLIP oncoprotein induces B cell transdifferentiation and tumorigenesis in mice
- PMID: 21339646
- PMCID: PMC3049379
- DOI: 10.1172/JCI44417
Kaposi sarcoma herpesvirus (KSHV) vFLIP oncoprotein induces B cell transdifferentiation and tumorigenesis in mice
Abstract
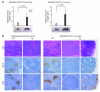
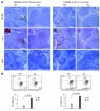
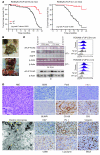
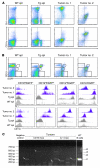
Kaposi sarcoma herpesvirus (KSHV) is specifically associated with Kaposi sarcoma (KS) and 2 B cell lymphoproliferative diseases, namely primary effusion lymphoma (PEL) and multicentric Castleman disease (MCD). KS, PEL, and MCD are largely incurable and poorly understood diseases most common in HIV-infected individuals. Here, we have revealed the role of viral FLICE-inhibitory protein (vFLIP) in the initiation of PEL and MCD by specifically expressing vFLIP at different stages of B cell differentiation in vivo. Mice showed MCD-like abnormalities and immunological defects including lack of germinal centers (GCs), impaired Ig class switching, and affinity maturation. In addition, they showed increased numbers of cells expressing cytoplasmic IgM-λ, a thus far enigmatic feature of the KSHV-infected cells in MCD. B cell-derived tumors arose at high incidence and displayed Ig gene rearrangement with downregulated expression of B cell-associated antigens, which are features of PEL. Interestingly, these tumors exhibited characteristics of transdifferentiation and acquired expression of histiocytic/dendritic cell markers. These results define immunological functions for vFLIP in vivo and reveal what we believe to be a novel viral-mediated tumorigenic mechanism involving B cell reprogramming. Additionally, the robust recapitulation of KSHV-associated diseases in mice provides a model to test inhibitors of vFLIP as potential anticancer agents.
Figures




Comment in
-
Unraveling virus-induced lymphomagenesis.J Clin Invest. 2011 Mar;121(3):838-41. doi: 10.1172/JCI46499. Epub 2011 Feb 21. J Clin Invest. 2011. PMID: 21339638 Free PMC article.
References
-
- Soulier J, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86(4):1276–1280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

