Persistence of immune responses after a single dose of Novartis meningococcal serogroup A, C, W-135 and Y CRM-197 conjugate vaccine (Menveo®) or Menactra® among healthy adolescents
- PMID: 21339701
- PMCID: PMC3060384
- DOI: 10.4161/hv.6.11.12849
Persistence of immune responses after a single dose of Novartis meningococcal serogroup A, C, W-135 and Y CRM-197 conjugate vaccine (Menveo®) or Menactra® among healthy adolescents
Abstract
The persistence of human bactericidal activity (hSBA) responses in adolescents was assessed 22 months after vaccination with one dose of Menveo® (MenACWY-CRM; Novartis) or Menactra® (MCV4) (sanofi pasteur). The proportion of subjects with hSBA titers ≥8 was significantly higher among recipients of MenACWY-CRM than MCV4 for serogroups A, W-135 and Y.
Trial registration: ClinicalTrials.gov NCT00856297.
Figures
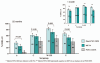
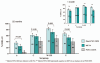
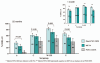
Similar articles
-
Antibody persistence and booster response of a quadrivalent meningococcal conjugate vaccine in adolescents.J Pediatr. 2014 Jun;164(6):1409-15.e4. doi: 10.1016/j.jpeds.2014.02.025. Epub 2014 Mar 20. J Pediatr. 2014. PMID: 24657122 Clinical Trial.
-
Phase III comparison of an investigational quadrivalent meningococcal conjugate vaccine with the licensed meningococcal ACWY conjugate vaccine in adolescents.Clin Infect Dis. 2009 Jul 1;49(1):e1-10. doi: 10.1086/599117. Clin Infect Dis. 2009. PMID: 19476428 Clinical Trial.
-
Antibody persistence and response to a booster dose of a quadrivalent conjugate vaccine for meningococcal disease in adolescents.Pediatr Infect Dis J. 2013 Apr;32(4):e170-7. doi: 10.1097/INF.0b013e318279ac38. Pediatr Infect Dis J. 2013. PMID: 23114372
-
Menveo®): a novel quadrivalent meningococcal CRM197 conjugate vaccine against serogroups A, C, W-135 and Y.Expert Rev Vaccines. 2011 Jan;10(1):21-33. doi: 10.1586/erv.10.147. Expert Rev Vaccines. 2011. PMID: 21162617 Review.
-
Persistence of the immune response after MenACWY-CRM vaccination and response to a booster dose, in adolescents, children and infants.Hum Vaccin Immunother. 2016 May 3;12(5):1300-10. doi: 10.1080/21645515.2015.1136040. Epub 2016 Feb 1. Hum Vaccin Immunother. 2016. PMID: 26829877 Free PMC article. Review.
Cited by
-
Meningococcal disease and vaccination in college students.Hum Vaccin Immunother. 2021 Nov 2;17(11):4675-4688. doi: 10.1080/21645515.2021.1973881. Epub 2021 Oct 6. Hum Vaccin Immunother. 2021. PMID: 34613863 Free PMC article. Review.
-
Cost-effectiveness analysis of vaccination strategies against meningococcal disease for children under nine years of age in China.Hum Vaccin Immunother. 2024 Dec 31;20(1):2313872. doi: 10.1080/21645515.2024.2313872. Epub 2024 Feb 13. Hum Vaccin Immunother. 2024. PMID: 38348600 Free PMC article.
-
Modeling protective meningococcal antibody responses and factors influencing antibody persistence following vaccination with MenAfriVac using machine learning.PLoS One. 2025 May 14;20(5):e0323384. doi: 10.1371/journal.pone.0323384. eCollection 2025. PLoS One. 2025. PMID: 40367245 Free PMC article.
-
Comparison of Immune Responses to Two Quadrivalent Meningococcal Conjugate Vaccines (CRM197 and Diphtheria Toxoid) in Healthy Adults.J Korean Med Sci. 2019 Jun 17;34(23):e169. doi: 10.3346/jkms.2019.34.e169. J Korean Med Sci. 2019. PMID: 31197986 Free PMC article. Clinical Trial.
-
Critical appraisal of a quadrivalent CRM(197) conjugate vaccine against meningococcal serogroups A, C W-135 and Y (Menveo) in the context of treatment and prevention of invasive disease.Infect Drug Resist. 2011;4:137-47. doi: 10.2147/IDR.S12716. Epub 2011 Jul 22. Infect Drug Resist. 2011. PMID: 21904459 Free PMC article.
References
-
- Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia and Neisseria meningitidis. Lancet. 2007;369:2196–2210. - PubMed
-
- Auckland C, Gray S, Borrow R, Andrews N, Goldblatt D, Ramsay M, et al. Clinical and immunologic risk factors for meningococcal C conjugate vaccine failure in the United Kingdom. J Infect Dis. 2006;194:1745–1752. - PubMed
-
- McVernon J, Mitchison NA, Moxon ER. T helper cells and efficacy of Haemophilus influenzae type b conjugate vaccination. Lancet Infect Dis. 2004;4:40–43. - PubMed
-
- Frasch CE, Borrow R, Donnelly J. Bactericidal antibody is the immunologic surrogate of protection against meningococcal disease. Vaccine. 2009;27:112–116. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
