Structure of dihydrochalcones and related derivatives and their scavenging and antioxidant activity against oxygen and nitrogen radical species
- PMID: 21339710
- PMCID: PMC6259755
- DOI: 10.3390/molecules16021749
Structure of dihydrochalcones and related derivatives and their scavenging and antioxidant activity against oxygen and nitrogen radical species
Abstract
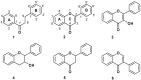
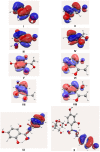
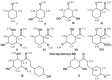
Quantum mechanical calculations at B3LYP/6-31G** level of theory were employed to obtain energy (E), ionization potential (IP), bond dissociation enthalpy (O-H BDE) and stabilization energies (DE(iso)) in order to infer the scavenging activity of dihydrochalcones (DHC) and structurally related compounds. Spin density calculations were also performed for the proposed antioxidant activity mechanism of 2,4,6-trihydroxyacetophenone (2,4,6-THA). The unpaired electron formed by the hydrogen abstraction from the phenolic hydroxyl group of 2,4,6-THA is localized on the phenolic oxygen at 2, 6, and 4 positions, the C₃ and C₆ carbon atoms at ortho positions, and the C₅ carbon atom at para position. The lowest phenolic oxygen contribution corresponded to the highest scavenging activity value. It was found that antioxidant activity depends on the presence of a hydroxyl at the C2 and C4 positions and that there is a correlation between IP and O-H BDE and peroxynitrite scavenging activity and lipid peroxidation. These results identified the pharmacophore group for DHC.
Figures


Similar articles
-
Density functional theory (DFT) study of edaravone derivatives as antioxidants.Int J Mol Sci. 2012;13(6):7594-7606. doi: 10.3390/ijms13067594. Epub 2012 Jun 20. Int J Mol Sci. 2012. PMID: 22837715 Free PMC article.
-
Antioxidant Properties of Kynurenines: Density Functional Theory Calculations.PLoS Comput Biol. 2016 Nov 18;12(11):e1005213. doi: 10.1371/journal.pcbi.1005213. eCollection 2016 Nov. PLoS Comput Biol. 2016. PMID: 27861556 Free PMC article.
-
Theoretical study on the structural and antioxidant properties of some recently synthesised 2,4,5-trimethoxy chalcones.Food Chem. 2015 Mar 15;171:89-97. doi: 10.1016/j.foodchem.2014.08.106. Epub 2014 Sep 4. Food Chem. 2015. PMID: 25308647
-
Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger.Curr Top Med Chem. 2002 Feb;2(2):181-97. doi: 10.2174/1568026023394443. Curr Top Med Chem. 2002. PMID: 11899100 Review.
-
Antioxidant properties of aminoethylcysteine ketimine decarboxylated dimer: a review.Int J Mol Sci. 2011;12(5):3072-84. doi: 10.3390/ijms12053072. Epub 2011 May 12. Int J Mol Sci. 2011. PMID: 21686170 Free PMC article. Review.
Cited by
-
Enolate-Forming Phloretin Pharmacophores: Hepatoprotection in an Experimental Model of Drug-Induced Toxicity.J Pharmacol Exp Ther. 2016 Jun;357(3):476-86. doi: 10.1124/jpet.115.231001. Epub 2016 Mar 30. J Pharmacol Exp Ther. 2016. PMID: 27029584 Free PMC article.
-
Evaluation of the antiradical activity of hyperjovinol-A utilizing donor-acceptor maps.J Mol Model. 2014 Jul;20(7):2337. doi: 10.1007/s00894-014-2337-y. J Mol Model. 2014. PMID: 25069138
-
Investigation of the antioxidant properties of hyperjovinol A through its Cu(II) coordination ability.J Mol Model. 2013 May;19(5):2127-42. doi: 10.1007/s00894-012-1684-9. Epub 2012 Dec 5. J Mol Model. 2013. PMID: 23212237
-
QSAR study of phenolic compounds and their anti-DPPH radical activity by discriminant analysis.Sci Rep. 2022 May 12;12(1):7860. doi: 10.1038/s41598-022-11925-y. Sci Rep. 2022. PMID: 35552494 Free PMC article.
-
Journey on Naphthoquinone and Anthraquinone Derivatives: New Insights in Alzheimer's Disease.Pharmaceuticals (Basel). 2021 Jan 5;14(1):33. doi: 10.3390/ph14010033. Pharmaceuticals (Basel). 2021. PMID: 33466332 Free PMC article. Review.
References
-
- Hertog M.G.L., Hollman P.C.H., Van de Putte B. Content of potentially anticarcinogenic flavonoids of tea infusions, wines and fruit juices. J. Agric. Food Chem. 1993;41:1242–1246.
-
- Calliste C., Bail J.L., Trouillas P., Pouget C., Habrioux G., Chulia A., Duroux J. Chalcones: Structural requirements for antioxidant, estrogenic and antiproliferative activities. Anticancer Res. 2001;21:3949–3956. - PubMed
-
- Mathiesen L., Malterud K.E., Sound R.B. Hydrogen Bond Formation as Basis For Radical Scavenging Activity: A Structure-Activity Study of C-Methylated Dihydrochalcones from Myrica gale and Structurally Related Acetophenones. Free Radical Biol. Med. 1997;22:307–315. doi: 10.1016/S0891-5849(96)00277-8. - DOI - PubMed
-
- Leopoldini M., Prieto Pitarch I., Russo N., Toscano M. Structure, conformation and electronic properties of apigenin, luteolin and taxifolin antioxidants. A first principle theoretical study. J. Phys. Chem. B. 2004;108:92–94. doi: 10.1021/jp035901j. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

