Diversity of phage-displayed libraries of peptides during panning and amplification
- PMID: 21339712
- PMCID: PMC6259649
- DOI: 10.3390/molecules16021776
Diversity of phage-displayed libraries of peptides during panning and amplification
Abstract
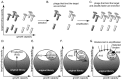
The amplification of phage-displayed libraries is an essential step in the selection of ligands from these libraries. The amplification of libraries, however, decreases their diversity and limits the number of binding clones that a screen can identify. While this decrease might not be a problem for screens against targets with a single binding site (e.g., proteins), it can severely hinder the identification of useful ligands for targets with multiple binding sites (e.g., cells). This review aims to characterize the loss in the diversity of libraries during amplification. Analysis of the peptide sequences obtained in several hundred screens of peptide libraries shows explicitly that there is a significant decrease in library diversity that occurs during the amplification of phage in bacteria. This loss during amplification is not unique to specific libraries: it is observed in many of the phage display systems we have surveyed. The loss in library diversity originates from competition among phage clones in a common pool of bacteria. Based on growth data from the literature and models of phage growth, we show that this competition originates from growth rate differences of only a few percent for different phage clones. We summarize the findings using a simple two-dimensional "phage phase diagram", which describes how the collapse of libraries, due to panning and amplification, leads to the identification of only a subset of the available ligands. This review also highlights techniques that allow elimination of amplification-induced losses of diversity, and how these techniques can be used to improve phage-display selection and enable the identification of novel ligands.
Figures







Similar articles
-
Deep sequencing analysis of phage libraries using Illumina platform.Methods. 2012 Sep;58(1):47-55. doi: 10.1016/j.ymeth.2012.07.006. Epub 2012 Jul 20. Methods. 2012. PMID: 22819855
-
Prospective identification of parasitic sequences in phage display screens.Nucleic Acids Res. 2014 Feb;42(3):1784-98. doi: 10.1093/nar/gkt1104. Epub 2013 Nov 11. Nucleic Acids Res. 2014. PMID: 24217917 Free PMC article.
-
Phage-displayed combinatorial peptide libraries in fusion to beta-lactamase as reporter for an accelerated clone screening: Potential uses of selected enzyme-linked affinity reagents in downstream applications.Comb Chem High Throughput Screen. 2010 Jan;13(1):75-87. doi: 10.2174/138620710790218258. Comb Chem High Throughput Screen. 2010. PMID: 20214576 Free PMC article.
-
Phage display: concept, innovations, applications and future.Biotechnol Adv. 2010 Nov-Dec;28(6):849-58. doi: 10.1016/j.biotechadv.2010.07.004. Epub 2010 Jul 23. Biotechnol Adv. 2010. PMID: 20659548 Review.
-
Designing scaffolds of peptides for phage display libraries.J Biosci Bioeng. 2005 May;99(5):448-56. doi: 10.1263/jbb.99.448. J Biosci Bioeng. 2005. PMID: 16233816 Review.
Cited by
-
Pulmonary Targeting of Adeno-associated Viral Vectors by Next-generation Sequencing-guided Screening of Random Capsid Displayed Peptide Libraries.Mol Ther. 2016 Jun;24(6):1050-1061. doi: 10.1038/mt.2016.62. Epub 2016 Mar 28. Mol Ther. 2016. PMID: 27018516 Free PMC article.
-
A method for rapid nanobody screening with no bias of the library diversity.iScience. 2024 Jan 18;27(2):108966. doi: 10.1016/j.isci.2024.108966. eCollection 2024 Feb 16. iScience. 2024. PMID: 38327779 Free PMC article.
-
Intra-domain phage display (ID-PhD) of peptides and protein mini-domains censored from canonical pIII phage display.Front Microbiol. 2015 Apr 28;6:340. doi: 10.3389/fmicb.2015.00340. eCollection 2015. Front Microbiol. 2015. PMID: 25972845 Free PMC article.
-
Selection of cell-type specific antibodies on tissue-sections using phage display.J Cell Mol Med. 2015 Aug;19(8):1939-48. doi: 10.1111/jcmm.12568. Epub 2015 Mar 26. J Cell Mol Med. 2015. PMID: 25808085 Free PMC article.
-
Propagation Capacity of Phage Display Peptide Libraries Is Affected by the Length and Conformation of Displayed Peptide.Molecules. 2023 Jul 10;28(14):5318. doi: 10.3390/molecules28145318. Molecules. 2023. PMID: 37513190 Free PMC article.
References
-
- Devlin J.J., Panganiban L.C., Devlin P.E. Random Peptide Libraries - a Source of Specific Protein-Binding Molecules. Science. 1990;249:404–406. - PubMed
-
- McCafferty J., Griffiths A.D., Winter G., Chiswell D.J. Phage Antibodies - Filamentous Phage Displaying Antibody Variable Domains. Nature. 1990;348:552–554. - PubMed
-
- Smith G.P. Filamentous Fusion Phage - Novel Expression Vectors That Display Cloned Antigens on the Virion Surface. Science. 1985;228:1315–1317. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

