Structure and stability of the spinach aquaporin SoPIP2;1 in detergent micelles and lipid membranes
- PMID: 21339815
- PMCID: PMC3038850
- DOI: 10.1371/journal.pone.0014674
Structure and stability of the spinach aquaporin SoPIP2;1 in detergent micelles and lipid membranes
Erratum in
- PLoS One. 2011;6(2). doi: 10.1371/annotation/589616b0-91e1-406c-8cf9-fe78ac08b8f6 doi: 10.1371/annotation/589616b0-91e1-406c-8cf9-fe78ac08b8f6
Abstract
Background: SoPIP2;1 constitutes one of the major integral proteins in spinach leaf plasma membranes and belongs to the aquaporin family. SoPIP2;1 is a highly permeable and selective water channel that has been successfully overexpressed and purified with high yields. In order to optimize reconstitution of the purified protein into biomimetic systems, we have here for the first time characterized the structural stability of SoPIP2;1.
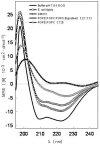
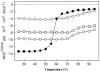
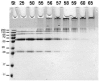
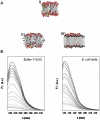
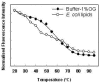
Methodology/principal finding: We have characterized the protein structural stability after purification and after reconstitution into detergent micelles and proteoliposomes using circular dichroism and fluorescence spectroscopy techniques. The structure of SoPIP2;1 was analyzed either with the protein solubilized with octyl-β-D-glucopyranoside (OG) or reconstituted into lipid membranes formed by E. coli lipids, diphytanoylphosphatidylcholine (DPhPC), or reconstituted into lipid membranes formed from mixtures of 1-palmitoyl-2-oleoyl-phosphatidylcholine (POPE), 1-palmitoyl-2oleoyl-phosphatidylethanolamine (POPE), 1-palmitoyl-2-oleoyl-phosphatidylserine (POPS), and ergosterol. Generally, SoPIP2;1 secondary structure was found to be predominantly α-helical in accordance with crystallographic data. The protein has a high thermal structural stability in detergent solutions, with an irreversible thermal unfolding occurring at a melting temperature of 58°C. Incorporation of the protein into lipid membranes increases the structural stability as evidenced by an increased melting temperature of up to 70°C.
Conclusion/significance: The results of this study provide insights into SoPIP2;1 stability in various host membranes and suggest suitable choices of detergent and lipid composition for reconstitution of SoPIP2;1 into biomimetic membranes for biotechnological applications.
Conflict of interest statement
Figures

References
-
- Engel A, Stahlberg H. Aquaglyceroporins: channel proteins with a conserved core, multiple functions, and variable surfaces. Int Rev Cytol. 2002;215:75–104. - PubMed
-
- Park JH, Saier MH., Jr Phylogenetic characterization of the MIP family of transmembrane channel proteins. J Membr Biol. 1996;153:171–180. - PubMed
-
- Kukulski W, Schenk AD, Johanson U, Braun T, de Groot BL, et al. The 5A structure of heterologously expressed plant aquaporin SoPIP2;1. J Mol Biol. 2005;350:611–616. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

