Acute Seizures in Old Age Leads to a Greater Loss of CA1 Pyramidal Neurons, an Increased Propensity for Developing Chronic TLE and a Severe Cognitive Dysfunction
- PMID: 21339903
- PMCID: PMC3041587
Acute Seizures in Old Age Leads to a Greater Loss of CA1 Pyramidal Neurons, an Increased Propensity for Developing Chronic TLE and a Severe Cognitive Dysfunction
Abstract
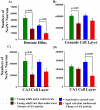
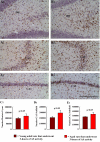
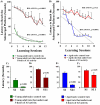
The aged population displays an enhanced risk for developing acute seizure (AS) activity. However, it is unclear whether AS activity in old age would result in a greater magnitude of hippocampal neurodegeneration and inflammation, and an increased predilection for developing chronic temporal lobe epilepsy (TLE) and cognitive dysfunction. Therefore, we addressed these issues in young-adult (5-months old) and aged (22-months old) F344 rats after three-hours of AS activity, induced through graded intraperitoneal injections of kainic acid (KA), and terminated through a diazepam injection. During the three-hours of AS activity, both young adult and aged groups exhibited similar numbers of stage-V motor seizures but the numbers of stage-IV motor seizures were greater in the aged group. In both age groups, three-hour AS activity induced degeneration of 50-55% of neurons in the dentate hilus, 22-32% of neurons in the granule cell layer and 49-52% neurons in the CA3 pyramidal cell layer without showing any interaction between the age and AS activity. However, degeneration of neurons in the CA1 pyramidal cell layer showed a clear interaction between the age and AS activity (12% in the young adult group and 56% in the aged group), suggesting that an advanced age makes the CA1 pyramidal neurons more susceptible to die with AS activity. The extent of inflammation measured through the numbers of activated microglial cells was similar between the two age groups. Interestingly, the predisposition for developing chronic TLE at 2-3 months after AS activity was 60% for young adult rats but 100% for aged rats. Moreover, both frequency & intensity of spontaneous recurrent seizures in the chronic phase after AS activity were 6-12 folds greater in aged rats than in young adult rats. Furthermore, aged rats lost their ability for spatial learning even in a scrupulous eleven-session water maze learning paradigm after AS activity, in divergence from young adult rats which retained the ability for spatial learning but had memory retrieval dysfunction after AS activity. Thus, AS activity in old age results in a greater loss of hippocampal CA1 pyramidal neurons, an increased propensity for developing robust chronic TLE, and a severe cognitive dysfunction.
Figures
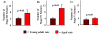



References
-
- Bhatnagar M, Cintra A, Chadi G, et al. Neurochemical changes in the hippocampus of the brown Norway rat during aging. Neurobiol Aging. 1997;18:319–327. - PubMed
-
- Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195:353–371. - PubMed
-
- Nicolle MM, Gonzalez J, Sugaya K, Baskerville KA, Bryan D, Lund K, Gallagher M, McKinney M. Signatures of hippocampal oxidative stress in aged spatial learning-impaired rodents. Neuroscience. 2001;107:415–431. - PubMed
-
- Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferationfactors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51:173–186. - PubMed
-
- Shetty AK, Rao MS, Hattiangady B, Zaman V, Shetty GA. Hippocampal neurotrophin levels after injury: Relationship to the age of the hippocampus at the time of injury. J Neurosci Res. 2004;78:520–532. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
