Secondary metabolites from a marine-derived endophytic fungus Penicillium chrysogenum QEN-24S
- PMID: 21339945
- PMCID: PMC3039470
- DOI: 10.3390/md9010059
Secondary metabolites from a marine-derived endophytic fungus Penicillium chrysogenum QEN-24S
Abstract
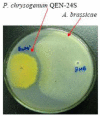
Penicillium chrysogenum QEN-24S, an endophytic fungus isolated from an unidentified marine red algal species of the genus Laurencia, displayed inhibitory activity against the growth of pathogen Alternaria brassicae in dual culture test. Chemical investigation of this fungal strain resulted in the isolation of four new (1-3 and 5) and one known (4) secondary metabolites. Their structures were identified as two polyketide derivatives penicitides A and B (1 and 2), two glycerol derivatives 2-(2,4-dihydroxy-6-methylbenzoyl)-glycerol (3) and 1-(2,4-dihydroxy-6-methylbenzoyl)- glycerol (4), and one monoterpene derivative penicimonoterpene (5). Penicitides A and B (1 and 2) feature a unique 10-hydroxy- or 7,10-dihydroxy-5,7-dimethylundecyl moiety substituting at C-5 of the α-tetrahydropyrone ring, which is not reported previously among natural products. Compound 5 displayed potent activity against the pathogen A. brassicae, while compound 1 exhibited moderate cytotoxic activity against the human hepatocellular liver carcinoma cell line.
Keywords: Penicillium chrysogenum; marine endophyte; secondary metabolites.
Figures

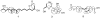
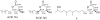

References
-
- Bugni TS, Ireland CM. Marine-derived fungi: A chemically and biologically diverse group of microorganisms. Nat Prod Rep. 2004;21:143–163. - PubMed
-
- Blunt JW, Copp BR, Hu WP, Munro MHG, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep. 2008;25:35–94. - PubMed
-
- Blunt JW, Copp BR, Hu WP, Munro MHG, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep. 2009;26:170–244. - PubMed
-
- Blunt JW, Copp BR, Munro MHG, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep. 2010;27:165–237. - PubMed
-
- Zhang Y, Li XM, Wang BG. Nigerasperones A–C, new monomeric and dimeric naphtho-γ-pyrones from a marine alga-derived endophytic fungus Aspergillus niger EN-13. J Antibiot. 2007;60:204–210. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous
