Diversity of nonribosomal peptide synthetases involved in the biosynthesis of lipopeptide biosurfactants
- PMID: 21339982
- PMCID: PMC3039948
- DOI: 10.3390/ijms12010141
Diversity of nonribosomal peptide synthetases involved in the biosynthesis of lipopeptide biosurfactants
Abstract
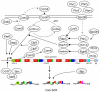
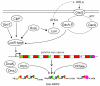
Lipopeptide biosurfactants (LPBSs) consist of a hydrophobic fatty acid portion linked to a hydrophilic peptide chain in the molecule. With their complex and diverse structures, LPBSs exhibit various biological activities including surface activity as well as anti-cellular and anti-enzymatic activities. LPBSs are also involved in multi-cellular behaviors such as swarming motility and biofilm formation. Among the bacterial genera, Bacillus (Gram-positive) and Pseudomonas (Gram-negative) have received the most attention because they produce a wide range of effective LPBSs that are potentially useful for agricultural, chemical, food, and pharmaceutical industries. The biosynthetic mechanisms and gene regulation systems of LPBSs have been extensively analyzed over the last decade. LPBSs are generally synthesized in a ribosome-independent manner with megaenzymes called nonribosomal peptide synthetases (NRPSs). Production of active-form NRPSs requires not only transcriptional induction and translation but also post-translational modification and assemblage. The accumulated knowledge reveals the versatility and evolutionary lineage of the NRPSs system. This review provides an overview of the structural and functional diversity of LPBSs and their different biosynthetic mechanisms in Bacillus and Pseudomonas, including both typical and unique systems. Finally, successful genetic engineering of NRPSs for creating novel lipopeptides is also discussed.
Keywords: lipopeptide biosurfactants (LPBSs); nonribosomal peptide synthetases (NRPSs); nonribosomal peptides.
Figures


References
-
- Georgiou G, Lin SC, Sharma MM. Surface-active compounds from microorganisms. Biotechnology (NY) 1992;10:60–65. - PubMed
-
- Arima K, Kakinuma A, Tamura G. Surfactin, a crystalline peptidelipid surfactant produced by Bacillus subtilis: isolation, characterization and its inhibition of fibrin clot formation. Biochem. Biophys. Res. Commun. 1968;31:488–494. - PubMed
-
- Nishikiori T, Naganawa H, Muraoka Y, Aoyagi T, Umezawa H. Plipastatins: new inhibitors of phospholipase A2, produced by Bacillus cereus BMG302-fF67. III. Structural elucidation of plipastatins. J. Antibiot (Tokyo) 1986;39:755–761. - PubMed
-
- Peypoux F, Pommier MT, Das BC, Besson F, Delcambe L, Michel G. Structures of bacillomycin D and bacillomycin L peptidolipid antibiotics from Bacillus subtilis. J. Antibiot (Tokyo) 1984;37:1600–1604. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

