The biochemical and cellular basis for nutraceutical strategies to attenuate neurodegeneration in Parkinson's disease
- PMID: 21340000
- PMCID: PMC3039966
- DOI: 10.3390/ijms12010506
The biochemical and cellular basis for nutraceutical strategies to attenuate neurodegeneration in Parkinson's disease
Abstract
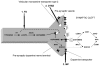
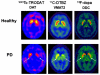
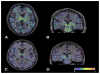
Future therapeutic intervention that could effectively decelerate the rate of degeneration within the substantia nigra pars compacta (SNc) could add years of mobility and reduce morbidity associated with Parkinson's disease (PD). Neurodegenerative decline associated with PD is distinguished by extensive damage to SNc dopaminergic (DAergic) neurons and decay of the striatal tract. While genetic mutations or environmental toxins can precipitate pathology, progressive degenerative succession involves a gradual decline in DA neurotransmission/synaptic uptake, impaired oxidative glucose consumption, a rise in striatal lactate and chronic inflammation. Nutraceuticals play a fundamental role in energy metabolism and signaling transduction pathways that control neurotransmission and inflammation. However, the use of nutritional supplements to slow the progression of PD has met with considerable challenge and has thus far proven unsuccessful. This review re-examines precipitating factors and insults involved in PD and how nutraceuticals can affect each of these biological targets. Discussed are disease dynamics (Sections 1 and 2) and natural substances, vitamins and minerals that could impact disease processes (Section 3). Topics include nutritional influences on α-synuclein aggregation, ubiquitin proteasome function, mTOR signaling/lysosomal-autophagy, energy failure, faulty catecholamine trafficking, DA oxidation, synthesis of toxic DA-quinones, o-semiquinones, benzothiazolines, hyperhomocyseinemia, methylation, inflammation and irreversible oxidation of neuromelanin. In summary, it is clear that future research will be required to consider the multi-faceted nature of this disease and re-examine how and why the use of nutritional multi-vitamin-mineral and plant-based combinations could be used to slow the progression of PD, if possible.
Keywords: Parkinson’s disease; neuromelanin; neuroprotective; nutrition; vitamins.
Figures
Similar articles
-
Dopaminergic Neuroprotection with Atremorine in Parkinson´s Disease.Curr Med Chem. 2018;25(39):5372-5388. doi: 10.2174/0929867325666180410100559. Curr Med Chem. 2018. PMID: 29637853 Review.
-
Toxic Feedback Loop Involving Iron, Reactive Oxygen Species, α-Synuclein and Neuromelanin in Parkinson's Disease and Intervention with Turmeric.Mol Neurobiol. 2021 Nov;58(11):5920-5936. doi: 10.1007/s12035-021-02516-5. Epub 2021 Aug 23. Mol Neurobiol. 2021. PMID: 34426907 Review.
-
Protective and toxic roles of dopamine in Parkinson's disease.J Neurochem. 2014 Jun;129(6):898-915. doi: 10.1111/jnc.12686. Epub 2014 Mar 18. J Neurochem. 2014. PMID: 24548101 Review.
-
Are Dopamine Oxidation Metabolites Involved in the Loss of Dopaminergic Neurons in the Nigrostriatal System in Parkinson's Disease?ACS Chem Neurosci. 2017 Apr 19;8(4):702-711. doi: 10.1021/acschemneuro.7b00034. Epub 2017 Mar 3. ACS Chem Neurosci. 2017. PMID: 28233992 Review.
-
The spatiotemporal changes in dopamine, neuromelanin and iron characterizing Parkinson's disease.Brain. 2021 Nov 29;144(10):3114-3125. doi: 10.1093/brain/awab191. Brain. 2021. PMID: 33978742 Free PMC article.
Cited by
-
Plant Foods Rich in Antioxidants and Human Cognition: A Systematic Review.Antioxidants (Basel). 2021 Apr 30;10(5):714. doi: 10.3390/antiox10050714. Antioxidants (Basel). 2021. PMID: 33946461 Free PMC article. Review.
-
Transition-State Analogues of Phenylethanolamine N-Methyltransferase.J Am Chem Soc. 2020 Aug 19;142(33):14222-14233. doi: 10.1021/jacs.0c05446. Epub 2020 Aug 7. J Am Chem Soc. 2020. PMID: 32702980 Free PMC article.
-
Alteration in biochemical parameters in the brain of transgenic Drosophila melanogaster model of Parkinson's disease exposed to apigenin.Integr Med Res. 2017 Sep;6(3):245-253. doi: 10.1016/j.imr.2017.04.003. Epub 2017 Apr 29. Integr Med Res. 2017. PMID: 28951838 Free PMC article.
-
Defective autophagy in Parkinson's disease: role of oxidative stress.Mol Neurobiol. 2012 Dec;46(3):639-61. doi: 10.1007/s12035-012-8318-1. Epub 2012 Aug 17. Mol Neurobiol. 2012. PMID: 22899187 Review.
-
Flavonoids as modulators of metabolic reprogramming in renal cell carcinoma (Review).Oncol Rep. 2024 Dec;52(6):167. doi: 10.3892/or.2024.8826. Epub 2024 Oct 18. Oncol Rep. 2024. PMID: 39422066 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

