A theoretical exploration of birhythmicity in the p53-Mdm2 network
- PMID: 21340030
- PMCID: PMC3038873
- DOI: 10.1371/journal.pone.0017075
A theoretical exploration of birhythmicity in the p53-Mdm2 network
Abstract
Experimental observations performed in the p53-Mdm2 network, one of the key protein modules involved in the control of proliferation of abnormal cells in mammals, revealed the existence of two frequencies of oscillations of p53 and Mdm2 in irradiated cells depending on the irradiation dose. These observations raised the question of the existence of birhythmicity, i.e. the coexistence of two oscillatory regimes for the same external conditions, in the p53-Mdm2 network which would be at the origin of these two distinct frequencies. A theoretical answer has been recently suggested by Ouattara, Abou-Jaoudé and Kaufman who proposed a 3-dimensional differential model showing birhythmicity to reproduce the two frequencies experimentally observed. The aim of this work is to analyze the mechanisms at the origin of the birhythmic behavior through a theoretical analysis of this differential model. To do so, we reduced this model, in a first step, into a 3-dimensional piecewise linear differential model where the Hill functions have been approximated by step functions, and, in a second step, into a 2-dimensional piecewise linear differential model by setting one autonomous variable as a constant in each domain of the phase space. We find that two features related to the phase space structure of the system are at the origin of the birhythmic behavior: the existence of two embedded cycles in the transition graph of the reduced models; the presence of a bypass in the orbit of the large amplitude oscillatory regime of low frequency. Based on this analysis, an experimental strategy is proposed to test the existence of birhythmicity in the p53-Mdm2 network. From a methodological point of view, this approach greatly facilitates the computational analysis of complex oscillatory behavior and could represent a valuable tool to explore mathematical models of biological rhythms showing sufficiently steep nonlinearities.
Conflict of interest statement
Figures


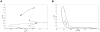

 which appears in the equation of Mc in Model 1 (in black) and its approximation into the step function
which appears in the equation of Mc in Model 1 (in black) and its approximation into the step function  defined as:
defined as:  if
if  and
and  if
if  (in red) for n = 6 and KMc = 0.4 nM. n is the Hill coefficient and characterizes the steepness of the Hill function.
(in red) for n = 6 and KMc = 0.4 nM. n is the Hill coefficient and characterizes the steepness of the Hill function.




References
-
- Goldbeter A. The molecular basis of periodic and chaotic behavior. Cambridge University Press; 1996. Biochemical Oscillations and Cellular Rythms.605
-
- Goldbeter A. Computational approaches to cellular rhythms. Nature. 2002;420:238–245. - PubMed
-
- Alamgir M, Epstein I. Birythmicity and compound oscillation in coupled chemical oscillators: chlorite-bromate-iodide system. J Am Chem Soc. 1983;105:2500–2501.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

