Randomized phase III study comparing the efficacy and safety of irinotecan plus S-1 with S-1 alone as first-line treatment for advanced gastric cancer (study GC0301/TOP-002)
- PMID: 21340666
- PMCID: PMC3056989
- DOI: 10.1007/s10120-011-0009-5
Randomized phase III study comparing the efficacy and safety of irinotecan plus S-1 with S-1 alone as first-line treatment for advanced gastric cancer (study GC0301/TOP-002)
Abstract
Background: Irinotecan hydrochloride and S-1, an oral fluoropyrimidine, have shown antitumor activity against advanced gastric cancer as single agents in phase I/II studies. The combination of irinotecan and S-1 (IRI-S) is also active against advanced gastric cancer. This study was conducted to compare the efficacy and safety of IRI-S versus S-1 monotherapy in patients with advanced or recurrent gastric cancer.
Methods: Patients were randomly assigned to oral S-1 (80 mg/m² daily for 28 days every 6 weeks) or oral S-1 (80 mg/m² daily for 21 days every 5 weeks) plus irinotecan (80 mg/m² by intravenous infusion on days 1 and 15 every 5 weeks) (IRI-S). The primary endpoint was overall survival. Secondary endpoints included the time to treatment failure, 1- and 2-year survival rates, response rate, and safety.
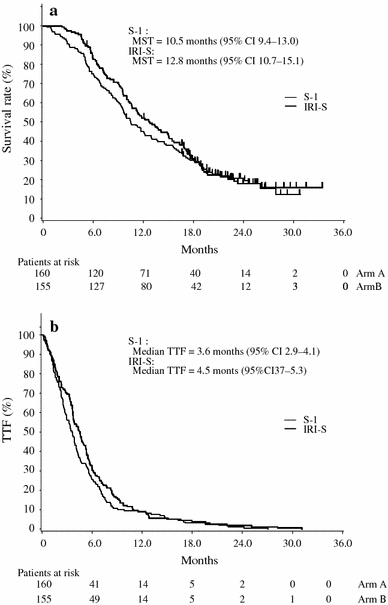
Results: The median survival time with IRI-S versus S-1 monotherapy was 12.8 versus 10.5 months (P = 0.233), time to treatment failure was 4.5 versus 3.6 months (P = 0.157), and the 1-year survival rate was 52.0 versus 44.9%, respectively. The response rate was significantly higher for IRI-S than for S-1 monotherapy (41.5 vs. 26.9%, P = 0.035). Neutropenia and diarrhea occurred more frequently with IRI-S, but were manageable. Patients treated with IRI-S received more courses of therapy at a relative dose intensity similar to that of S-1 monotherapy.
Conclusions: Although IRI-S achieved longer median survival than S-1 monotherapy and was well tolerated, it did not show significant superiority in this study.
Figures


References
-
- Foundation for Promotion of Cancer Research. Cancer Statistics in Japan 2007. Available from URL http://ganjoho.ncc.go.jp/public/statistics/backnumber/2007_en.html. Accessed June 21, 2010.
-
- Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E, et al. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol. 2000;18:2648–2657. - PubMed
-
- Dank M, Zaluski J, Barone C, Valvere V, Yalcin S, Peschel C, et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol. 2008;19:1450–1457. doi: 10.1093/annonc/mdn166. - DOI - PubMed
-
- Ajani JA, Moiseyenko VM, Tjulandin S, Majilis A, Constenla M, Boni C, et al. Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal adenocarcinoma: the V-325 Study Group. J Clin Oncol. 2007;25:3205–3209. doi: 10.1200/JCO.2006.10.4968. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

