Enantioselective synthesis of homoallylic amines through reactions of (pinacolato)allylborons with aryl-, heteroaryl-, alkyl-, or alkene-substituted aldimines catalyzed by chiral C1-symmetric NHC-Cu complexes
- PMID: 21341657
- PMCID: PMC3059089
- DOI: 10.1021/ja200311n
Enantioselective synthesis of homoallylic amines through reactions of (pinacolato)allylborons with aryl-, heteroaryl-, alkyl-, or alkene-substituted aldimines catalyzed by chiral C1-symmetric NHC-Cu complexes
Abstract
A catalytic method for enantioselective synthesis of homoallylamides through Cu-catalyzed reactions of stable and easily accessible (pinacolato)allylborons with aryl-, heteroaryl-, alkyl-, or alkenyl-substituted N-phosphinoylimines is disclosed. Transformations are promoted by 1-5 mol % of readily accessible NHC-Cu complexes, derived from C(1)-symmetric imidazolinium salts, which can be prepared in multigram quantities in four steps from commercially available materials. Allyl additions deliver the desired products in up to quantitative yield and 98.5:1.5 enantiomeric ratio and are amenable to gram-scale operations. A mechanistic model accounting for the observed selectivity levels and trends is proposed.
Figures

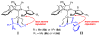

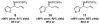

References
-
-
For general reviews that cover allyl additions to imines, see: Yamamoto Y, Asao N. Chem Rev. 1993;93:2207.Enders D, Reinhold U. Tetrahedron: Asymmetry. 1997;8:1895.Bloch R. Chem Rev. 1998;98:1407.Kobayashi S, Ishitani H. Chem Rev. 1999;99:1069.
-
-
-
For examples involving enantiomerically enriched allylboron reagents, see: With N-trimethylsilaimines: Itsuno S, Watanabe K, Ito K, El-Shehawy AA, Sarhan AA. Angew Chem, Int Ed Engl. 1997;36:109.With aldimine-trialkylborane adducts: Ramachandran PV, Biswas D. Org Lett. 2007;9:3025.With cyclic imines: Wu TR, Ching JM. J Am Chem Soc. 2006;128:9646.For transformations with enantiomerically enriched allylsilane reagents, see: With N-acylhydrazones: Berger R, Rabbat PMA, Leighton JL. J Am Chem Soc. 2003;125:9596.With o-anisidylimines: Rabbat PMA, Corey Valdez S, Leighton JL. Org Lett. 2006;8:6119.With a range of heterocyclic aldimines: Perl NR, Leighton JL. Org Lett. 2007;9:3699.With N-heteroaryl hydrazones: Feske MI, Santanilla AB, Leighton JL. Org Lett. 2010;12:688.
-
-
-
For example, see: Cogan DA, Liu G, Ellman J. Tetrahedron Lett. 1999;55:8883.Miyabe H, Yamaoka Y, Naito T, Takemoto Y. J Org Chem. 2004;69:1415.Vilaivan T, Winotapan C, Banphavichit V, Shinada T, Ohfune Y. J Org Chem. 2005;70:3464.Friestad GK, Korapala CS, Ding H. J Org Chem. 2006;71:281.Grigg R, McGaffrey S, Sridharan V, Fishwick CWG, Kilner C, Korn S, Bailey K, Blacker J. Tetrahedron. 2006;62:12159.Sun XW, Liu M, Xu MH, Lin GQ. Org Lett. 2008;10:1259.Reddy LR, Hu B, Prashad M, Prasad K. Org Lett. 2008;10:3109.Delaye PO, Pradhan TK, Lambert E, Vasse JL, Szymoniak J. Eur J Org Chem. 2010:3395.For a review, see: Alvaro G, Savoia D. Synlett. 2002:651.
-
-
-
For Cu-catalyzed allyl additions to α-amino esters, see: With allylstannanes: Fang X, Johannsen M, Yao S, Gathergood N, Hazell RG, Jørgensen KA. J Org Chem. 1999;64:4844.With alkenes: Ferraris D, Young B, Cox C, Dudding T, Drury WJ, III, Ryzhkov L, Taggi AE, Lectka T. J Am Chem Soc. 2002;124:67.With allylsilanes: Kiyohara H, Nakamura Y, Matsubara R, Kobayashi S. Angew Chem, Int Ed. 2006;45:1615.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

