Effects of selective neonatal hippocampal lesions on tests of object and spatial recognition memory in monkeys
- PMID: 21341885
- PMCID: PMC3072249
- DOI: 10.1037/a0022539
Effects of selective neonatal hippocampal lesions on tests of object and spatial recognition memory in monkeys
Abstract
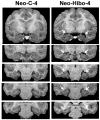
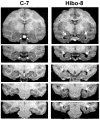
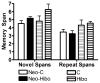
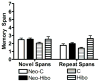
Earlier studies in monkeys have reported mild impairment in recognition memory after nonselective neonatal hippocampal lesions. To assess whether the memory impairment could have resulted from damage to cortical areas adjacent to the hippocampus, we tested adult monkeys with neonatal focal hippocampal lesions and sham-operated controls in three recognition tasks: delayed nonmatching-to-sample, object memory span, and spatial memory span. Further, to rule out that normal performance on these tasks may relate to functional sparing following neonatal hippocampal lesions, we tested adult monkeys that had received the same focal hippocampal lesions in adulthood and their controls in the same three memory tasks. Both early and late onset focal hippocampal damage did not alter performance on any of the three tasks, suggesting that damage to cortical areas adjacent to the hippocampus was likely responsible for the recognition impairment reported by the earlier studies. In addition, given that animals with early and late onset hippocampal lesions showed object and spatial recognition impairment when tested in a visual paired comparison task, the data suggest that not all object and spatial recognition tasks are solved by hippocampal-dependent memory processes. The current data may not only help explain the neural substrate for the partial recognition memory impairment reported in cases of developmental amnesia, but they are also clinically relevant given that the object and spatial memory tasks used in monkeys are often translated to investigate memory functions in several populations of human infants and children in which dysfunction of the hippocampus is suspected.
(PsycINFO Database Record (c) 2011 APA, all rights reserved).
Figures




Similar articles
-
Development of memory for spatial locations and object/place associations in infant rhesus macaques with and without neonatal hippocampal lesions.J Int Neuropsychol Soc. 2013 Nov;19(10):1053-64. doi: 10.1017/S1355617713000799. Epub 2013 Jul 23. J Int Neuropsychol Soc. 2013. PMID: 23880255 Free PMC article.
-
Developmental trajectory of object recognition memory in infant rhesus macaques with and without neonatal hippocampal lesions.J Neurosci. 2010 Jul 7;30(27):9157-65. doi: 10.1523/JNEUROSCI.0022-10.2010. J Neurosci. 2010. PMID: 20610749 Free PMC article.
-
Neonatal aspiration lesions of the hippocampal formation impair visual recognition memory when assessed by paired-comparison task but not by delayed nonmatching-to-sample task.Hippocampus. 1999;9(6):609-16. doi: 10.1002/(SICI)1098-1063(1999)9:6<609::AID-HIPO1>3.0.CO;2-A. Hippocampus. 1999. PMID: 10641753
-
Perspectives on object-recognition memory following hippocampal damage: lessons from studies in rats.Behav Brain Res. 2001 Dec 14;127(1-2):159-81. doi: 10.1016/s0166-4328(01)00367-9. Behav Brain Res. 2001. PMID: 11718890 Review.
-
Varieties of human spatial memory: a meta-analysis on the effects of hippocampal lesions.Brain Res Brain Res Rev. 2001 Jul;35(3):295-303. doi: 10.1016/s0165-0173(01)00058-3. Brain Res Brain Res Rev. 2001. PMID: 11423159 Review.
Cited by
-
Preserved visual memory and relational cognition performance in monkeys with selective hippocampal lesions.Sci Adv. 2020 Jul 17;6(29):eaaz0484. doi: 10.1126/sciadv.aaz0484. eCollection 2020 Jul. Sci Adv. 2020. PMID: 32832615 Free PMC article.
-
Measuring infant memory: Utility of the visual paired-comparison test paradigm for studies in developmental neurotoxicology.Neurotoxicol Teratol. 2012 Sep-Oct;34(5):473-80. doi: 10.1016/j.ntt.2012.06.003. Epub 2012 Jun 30. Neurotoxicol Teratol. 2012. PMID: 22750243 Free PMC article. Review.
-
Nonnavigational spatial memory performance is unaffected by hippocampal damage in monkeys.Hippocampus. 2019 Feb;29(2):93-101. doi: 10.1002/hipo.23013. Epub 2018 Sep 2. Hippocampus. 2019. PMID: 30069946 Free PMC article.
-
Stimulus similarity and encoding time influence incidental recognition memory in adult monkeys with selective hippocampal lesions.Learn Mem. 2011 Feb 25;18(3):170-80. doi: 10.1101/lm.2076811. Print 2011. Learn Mem. 2011. PMID: 21357439 Free PMC article.
-
Differential responses toward conditioned and unconditioned stimuli, but decreased hypothalamic-pituitary-adrenal axis responsiveness in neonatal hippocampal lesioned monkeys.Dev Cogn Neurosci. 2022 Dec;58:101165. doi: 10.1016/j.dcn.2022.101165. Epub 2022 Oct 17. Dev Cogn Neurosci. 2022. PMID: 36270099 Free PMC article.
References
-
- Bachevalier J, Beauregard M, Alvarado MC. Long-term effects of neonatal damage to the hippocampal formation and amygdaloid complex on object discrimination and object recognition in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 1999;113(6):1127–1151. - PubMed
-
- Bachevalier J, Meunier M. Cerebral ischemia: are the memory deficits associated with hippocampal cell loss? Hippocampus. 1996;6(5):553–560. - PubMed

