Mesenchymal stromal cells from neonatal tracheal aspirates demonstrate a pattern of lung-specific gene expression
- PMID: 21341990
- PMCID: PMC3202893
- DOI: 10.1089/scd.2010.0494
Mesenchymal stromal cells from neonatal tracheal aspirates demonstrate a pattern of lung-specific gene expression
Abstract
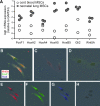
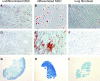
We have previously isolated mesenchymal stromal cells (MSCs) from the tracheal aspirates of premature neonates with respiratory distress. Although isolation of MSCs correlates with the development of bronchopulmonary dysplasia, the physiologic role of these cells remains unclear. To address this, we further characterized the cells, focusing on the issues of gene expression, origin, and cytokine expression. Microarray comparison of early passage neonatal lung MSC gene expression to cord blood MSCs and human fetal and neonatal lung fibroblast lines demonstrated that the neonatal lung MSCs differentially expressed 971 gene probes compared with cord blood MSCs, including the transcription factors Tbx2, Tbx3, Wnt5a, FoxF1, and Gli2, each of which has been associated with lung development. Compared with lung fibroblasts, 710 gene probe transcripts were differentially expressed by the lung MSCs, including IL-6 and IL-8/CXCL8. Differential chemokine expression was confirmed by protein analysis. Further, neonatal lung MSCs exhibited a pattern of Hox gene expression distinct from cord blood MSCs but similar to human fetal lung fibroblasts, consistent with a lung origin. On the other hand, limiting dilution analysis showed that fetal lung fibroblasts form colonies at a significantly lower rate than MSCs, and fibroblasts failed to undergo differentiation along adipogenic, osteogenic, and chondrogenic lineages. In conclusion, MSCs isolated from neonatal tracheal aspirates demonstrate a pattern of lung-specific gene expression, are distinct from lung fibroblasts, and secrete pro-inflammatory cytokines.
Figures



References
-
- Jobe AH. Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. - PubMed
-
- Hussain NA. Siddiqui NH. Stocker JR. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol. 1998;29:710–717. - PubMed
-
- Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol. 2003;8:73–81. - PubMed
-
- Bhatt AJ. Pryhuber GS. Huyck H. Watkins RH. Metlay LA. Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;164:1971–1980. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

