The use of light/chemically hardened polymethylmethacrylate, polyhydroxyethylmethacrylate, and calcium hydroxide graft material in combination with polyanhydride around implants in minipigs: part I: immediate stability and function
- PMID: 21342001
- PMCID: PMC4642450
- DOI: 10.1902/jop.2011.100617
The use of light/chemically hardened polymethylmethacrylate, polyhydroxyethylmethacrylate, and calcium hydroxide graft material in combination with polyanhydride around implants in minipigs: part I: immediate stability and function
Abstract
Background: The present study is designed as a proof-of-concept study to evaluate light/chemical hardening technology and a newly formulated polymethylmethacrylate, polyhydroxyethylmethacrylate, and calcium hydroxide (PPCH) plus polyanhydride (PA) (PPCH-PA) composite graft material as a bone substitute compared to positive and negative controls in a minipig model.
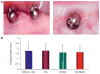
Methods: PPCH-PA (composite graft); PPCH alone (positive control), PA alone (positive control), and no graft (negative control) were compared. Four mandibular premolar teeth per quadrant were extracted; a total of 48 implants were placed into sockets in three minipigs. Abutments were placed protruding into the oral cavity 4 mm in height for immediate loading. Crestal areas and intrabony spaces were filled with PPCH-PA, PPCH, or PA using a three-phase delivery system in which all graft materials were hardened by a light cure. In the negative control group, implant sites were left untreated. At 12 weeks, block sections containing implants were obtained. Evaluations included periodontal probing, pullout-force load, and stability measurements to determine implant stability, radiographs to examine bone levels, and scanning electron microscopy (SEM)-energy-dispersed spectroscopy to determine bone-to-implant contact.
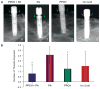
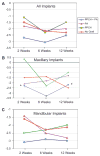
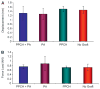
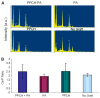
Results: Probing measurements did not reveal any pathologic pocket formation or bone loss. Radiographs revealed that immediate implant placement and loading resulted in bone at or slightly apical to the first thread of the implant in all groups at 12 weeks. Stability test values showed a relative clinical stability for all implants (range: -7 to +1); however, implants augmented with PPCH-PA exhibited a statistically significantly greater stability compared to all other groups (P <0.05). The newly formed bone in PPCH-PA-treated sites was well organized with less marrow spaces and well-distributed osteocytes. SEM revealed a tighter implant-socket interface in the PPCH-PA group compared to other groups with reduced microfissures and implant-bone interface fractures during pullout testing, whereas implants treated with PA or no graft showed ≈ 10-μm microfissures between the implant and bone with fractures of the intrathread bone.
Conclusions: The newly formulated chemically hardened graft material PPCH-PA was useful in immediate implant placement after tooth extraction and resulted in greater stability and a well-organized implant-bone interface with immediate loading, especially in those areas where cancellous bone was present. The results of this proof-of-concept study warranted further research investigating different healing times and longer durations.
Figures


Similar articles
-
The use of light/chemically hardened polymethylmethacrylate, polyhydroxylethylmethacrylate, and calcium hydroxide graft material in combination with polyanhydride around implants and extraction sockets in minipigs: Part II: histologic and micro-CT evaluations.J Periodontol. 2014 Sep;85(9):1230-9. doi: 10.1902/jop.2014.120424. Epub 2014 Feb 6. J Periodontol. 2014. PMID: 24502615 Free PMC article.
-
Evaluation of a new experimental model to study bone healing after ridge expansion with simultaneous implant placement--a pilot study in minipigs.Clin Oral Implants Res. 2014 Nov;25(11):1265-1272. doi: 10.1111/clr.12265. Epub 2013 Sep 18. Clin Oral Implants Res. 2014. PMID: 24102886
-
Alveolar ridge augmentation using anodized implants coated with Escherichia coli-derived recombinant human bone morphogenetic protein 2.Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011 Jul;112(1):42-9. doi: 10.1016/j.tripleo.2010.09.063. Epub 2010 Dec 30. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011. PMID: 21194992
-
The minipig intraoral dental implant model: A systematic review and meta-analysis.PLoS One. 2022 Feb 28;17(2):e0264475. doi: 10.1371/journal.pone.0264475. eCollection 2022. PLoS One. 2022. PMID: 35226690 Free PMC article.
-
Bone graft substitutes and dental implant stability in immediate implant surgery: a systematic review and meta-analysis.Evid Based Dent. 2025 Mar;26(1):70. doi: 10.1038/s41432-024-01077-5. Epub 2024 Nov 11. Evid Based Dent. 2025. PMID: 39528756
Cited by
-
Ethical guidelines, animal profile, various animal models used in periodontal research with alternatives and future perspectives.J Indian Soc Periodontol. 2016 Jul-Aug;20(4):360-368. doi: 10.4103/0972-124X.186931. J Indian Soc Periodontol. 2016. PMID: 28298815 Free PMC article. Review.
-
Animal models for periodontal regeneration and peri-implant responses.Periodontol 2000. 2015 Jun;68(1):66-82. doi: 10.1111/prd.12052. Periodontol 2000. 2015. PMID: 25867980 Free PMC article. Review.
-
The use of light/chemically hardened polymethylmethacrylate, polyhydroxylethylmethacrylate, and calcium hydroxide graft material in combination with polyanhydride around implants and extraction sockets in minipigs: Part II: histologic and micro-CT evaluations.J Periodontol. 2014 Sep;85(9):1230-9. doi: 10.1902/jop.2014.120424. Epub 2014 Feb 6. J Periodontol. 2014. PMID: 24502615 Free PMC article.
-
Immediate implant placement and simultaneous bone grafting with bone cement in extraction sockets: A systematic review.Saudi Dent J. 2024 Aug;36(8):1051-1057. doi: 10.1016/j.sdentj.2024.05.011. Epub 2024 May 24. Saudi Dent J. 2024. PMID: 39176154 Free PMC article. Review.
-
Proresolving nanomedicines activate bone regeneration in periodontitis.J Dent Res. 2015 Jan;94(1):148-56. doi: 10.1177/0022034514557331. Epub 2014 Nov 11. J Dent Res. 2015. PMID: 25389003 Free PMC article.
References
-
- Albrektsson T, Brånemark PI, Hansson HA, Lindström J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981;52:155–170. - PubMed
-
- Brunski JB, Moccia AF, Jr, Pollack SR, Korostoff E, Trachtenberg DI. The influence of functional use of endosseous dental implants on the tissue-implant interface. I. Histological aspects. J Dent Res. 1979;58:1953–1969. - PubMed
-
- Brånemark PI, Hansson BO, Adell R, et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl. 1977;16:1–132. - PubMed
-
- Brånemark PI, Engstrand P, Ohrnell LO, et al. Brånemark kovum: A new treatment concept for rehabilitation of the edentulous mandible. Preliminary results from a prospective clinical follow-up study. Clin Implant Dent Relat Res. 1999;1:2–16. - PubMed
-
- Becker W, Becker BE, Huffstetlert S. Early functional loading at 5 days for Brånemark implants placed into edentulous mandibles: A prospective, open-ended, longitudinal study. J Periodontol. 2003;74:695–702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

