Syntaxin 11 marks a distinct intracellular compartment recruited to the immunological synapse of NK cells to colocalize with cytotoxic granules
- PMID: 21342435
- PMCID: PMC3823099
- DOI: 10.1111/j.1582-4934.2011.01280.x
Syntaxin 11 marks a distinct intracellular compartment recruited to the immunological synapse of NK cells to colocalize with cytotoxic granules
Abstract
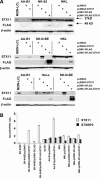
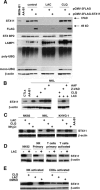
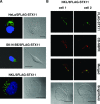
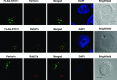
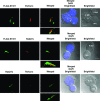
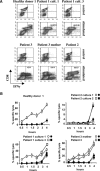
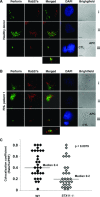
The syntaxin 11 (STX11) gene is mutated in a proportion of patients with familial haemophagocytic lymphohistiocytosis (FHL) and exocytosis of cytotoxic granules is impaired in STX11-deficient NK cells. However, the subcellular localization, regulation of expression and molecular function of STX11 in NK cells and other cytotoxic lymphocytes remain unknown. Here we demonstrate that STX11 expression is strictly controlled by several mechanisms in a cell-type-specific manner and that the enzymatic activity of the proteasome is required for STX11 expression in NK cells. In resting NKL cells, STX11 was localized in the cation-dependent mannose-6-phosphate receptor (CD-M6PR)-containing compartment, which was clearly distinct from cytotoxic granules or Rab27a-expressing vesicles. These subcellular structures appeared to fuse at the contact area with NK-sensitive target cells as demonstrated by partial colocalization of STX11 with perforin and Rab27a. Although STX11-deficent allo-specific cytotoxic T-lymphocytes efficiently lysed target cells and released cytotoxic granules, they exhibited a significantly lower extent of spontaneous association of perforin with Rab27a as compared with STX11-expressing T cells. Thus, our results suggest that STX11 promotes the fusion of Rab27a-expressing vesicles with cytotoxic granules and reveal an additional level of complexity in the spatial/temporal segregation of subcellular structures participating in the process of granule-mediated cytotoxicity.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures
Similar articles
-
Different NK cell-activating receptors preferentially recruit Rab27a or Munc13-4 to perforin-containing granules for cytotoxicity.Blood. 2009 Nov 5;114(19):4117-27. doi: 10.1182/blood-2009-06-225359. Epub 2009 Aug 24. Blood. 2009. PMID: 19704116
-
Syntaxin11 serves as a t-SNARE for the fusion of lytic granules in human cytotoxic T lymphocytes.Eur J Immunol. 2014 Feb;44(2):573-84. doi: 10.1002/eji.201344011. Epub 2013 Dec 16. Eur J Immunol. 2014. PMID: 24227526
-
A unique SNARE machinery for exocytosis of cytotoxic granules and platelets granules.Mol Membr Biol. 2015;32(4):120-6. doi: 10.3109/09687688.2015.1079934. Mol Membr Biol. 2015. PMID: 26508555 Review.
-
Comparison of primary human cytotoxic T-cell and natural killer cell responses reveal similar molecular requirements for lytic granule exocytosis but differences in cytokine production.Blood. 2013 Feb 21;121(8):1345-56. doi: 10.1182/blood-2012-07-442558. Epub 2013 Jan 2. Blood. 2013. PMID: 23287865
-
Familial hemophagocytic lymphohistiocytosis: a model for understanding the human machinery of cellular cytotoxicity.Cell Mol Life Sci. 2012 Jan;69(1):29-40. doi: 10.1007/s00018-011-0835-y. Epub 2011 Oct 12. Cell Mol Life Sci. 2012. PMID: 21990010 Free PMC article. Review.
Cited by
-
Human immunodeficiency syndromes affecting human natural killer cell cytolytic activity.Front Immunol. 2014 Jan 21;5:2. doi: 10.3389/fimmu.2014.00002. eCollection 2014. Front Immunol. 2014. PMID: 24478771 Free PMC article. Review.
-
The biogenesis of lysosomes and lysosome-related organelles.Cold Spring Harb Perspect Biol. 2014 Sep 2;6(9):a016840. doi: 10.1101/cshperspect.a016840. Cold Spring Harb Perspect Biol. 2014. PMID: 25183830 Free PMC article. Review.
-
PBRM1 and the glycosylphosphatidylinositol biosynthetic pathway promote tumor killing mediated by MHC-unrestricted cytotoxic lymphocytes.Sci Adv. 2020 Nov 27;6(48):eabc3243. doi: 10.1126/sciadv.abc3243. Print 2020 Nov. Sci Adv. 2020. PMID: 33246952 Free PMC article.
-
Genetic dissection of NK cell responses.Front Immunol. 2013 Jan 18;3:425. doi: 10.3389/fimmu.2012.00425. eCollection 2012. Front Immunol. 2013. PMID: 23346087 Free PMC article.
-
Synaptobrevin2 is the v-SNARE required for cytotoxic T-lymphocyte lytic granule fusion.Nat Commun. 2013;4:1439. doi: 10.1038/ncomms2467. Nat Commun. 2013. PMID: 23385584
References
-
- Ericson K, Fadeel B, Henter JI. Genetics and pathogenesis of hemophagocytic lymphohistiocytosis. In: Egeler RM, Weitzman S, editors. Histiocytic disorders of children and adults. Cambridge: Cambridge Press; 2005. pp. 337–52.
-
- Henter JI, Tondini C, Pritchard J. Histiocyte disorders. Crit Rev Oncol Hematol. 2004;50:157–74. - PubMed
-
- Verbsky JW, Grossman WJ. Hemophagocytic lymphohistiocytosis: diagnosis, pathophysiology, treatment, and future perspectives. Ann Med. 2006;38:20–31. - PubMed
-
- Feldmann J, Callebaut I, Raposo G, et al. Munc13–4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115:461–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

