Hsp90/Cdc37 chaperone/co-chaperone complex, a novel junction anticancer target elucidated by the mode of action of herbal drug Withaferin A
- PMID: 21342561
- PMCID: PMC3044286
- DOI: 10.1186/1471-2105-12-S1-S30
Hsp90/Cdc37 chaperone/co-chaperone complex, a novel junction anticancer target elucidated by the mode of action of herbal drug Withaferin A
Abstract
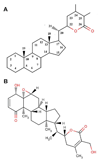
Background: HSPs (Heat shock proteins) are highly conserved ubiquitous proteins among species which are involved in maintaining appropriate folding and conformation of other proteins and are thus referred to as molecular chaperones. Hsp90 (Heat-shock protein 90 kDa) is one of a group of molecular chaperones responsible for managing protein folding and quality control in cell environment. However it is also involved in the maturation and stabilization of a wide range of oncogenic client proteins which are crucial for oncogenesis and malignant progression. Hsp90 requires a series of co-chaperones to assemble into a super-chaperone complex for its function. These co-chaperones bind and leave the complex at various stages to regulate the chaperoning process. Arresting the chaperone cycle at these stages by targeting different co-chaperone/Hsp90 interactions seems to be quite a viable alternative and is likely to achieve similar consequences as that of Hsp90 direct inhibition with added favors of high specificity and reduced side effect profile. The study conducted here is an attempt to explore the potential of Withania somnifera's major constituent WA (Withaferin A) in attenuating the Hsp90/Cdc37 chaperone/co-chaperone interactions for enhanced tumor arresting activity and to elucidate the underlying mode of action using computational approaches.
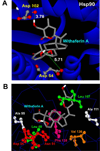
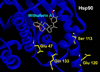
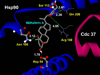
Results: Formation of active Hsp90/Cdc37 complex is one of the essential steps for facilitation of chaperone client interaction, non-assembly of which can lead to prevention of the chaperone-client association resulting in apoptosis of tumor cells. From our flexible docking analysis of WA into active Hsp90/Cdc37 complex in which key interfacing residues of the complex were kept flexible, disruption of the active association complex can be discerned. While docking of WA into segregated Hsp90 leaves the interface residues untouched. Thus the molecular docking analysis of WA into Hsp90 and active Hsp90/Cdc37 complex conducted in this study provides significant evidence in support of the proposed mechanism of chaperone assembly suppression by inhibition or disruption of active Hsp90/Cdc37 complex formation being accounted by non-assembly of the catalytically active Hsp90/Cdc37 complex. Results from the molecular dynamics simulations in water show that the trajectories of the protein complexed with ligand WA are stable over a considerably long time period of 4 ns, with the energies of the complex being lowered in comparison to the un-docked association complex, suggesting the thermodynamic stability of WA complexed Hsp90/Cdc37.
Conclusions: The molecular chaperone Hsp90 has been a promising target for cancer therapy. Cancer is a disease marked by genetic instability. Thus specific inhibition of individual proteins or signalling pathways holds a great potential for subversion of this genetic plasticity of cancers. This study is a step forward in this direction. Our computational analysis provided a rationalization to the ability of naturally occurring WA to alter the chaperone signalling pathway. The large value of binding energy involved in binding of WA to the active Hsp90/Cdc37 complex consolidates the thermodynamic stability of the binding. Our docking results obtained substantiate the hypothesis that WA has the potential to inhibit the association of chaperone (Hsp90) to its co-chaperone (Cdc37) by disrupting the stability of attachment of Hsp90 to Cdc37. Conclusively our results strongly suggest that withaferin A is a potent anticancer agent as ascertained by its potent Hsp90-client modulating capability.
Figures
Similar articles
-
Targeting the Hsp90-Cdc37-client protein interaction to disrupt Hsp90 chaperone machinery.J Hematol Oncol. 2018 Apr 27;11(1):59. doi: 10.1186/s13045-018-0602-8. J Hematol Oncol. 2018. PMID: 29699578 Free PMC article. Review.
-
Inhibition of the NEMO/IKKβ association complex formation, a novel mechanism associated with the NF-κB activation suppression by Withania somnifera's key metabolite withaferin A.BMC Genomics. 2010 Dec 2;11 Suppl 4(Suppl 4):S25. doi: 10.1186/1471-2164-11-S4-S25. BMC Genomics. 2010. PMID: 21143809 Free PMC article.
-
Blocking the chaperone kinome pathway: mechanistic insights into a novel dual inhibition approach for supra-additive suppression of malignant tumors.Biochem Biophys Res Commun. 2011 Jan 7;404(1):498-503. doi: 10.1016/j.bbrc.2010.12.010. Epub 2010 Dec 6. Biochem Biophys Res Commun. 2011. PMID: 21144839
-
Atomistic simulations and network-based modeling of the Hsp90-Cdc37 chaperone binding with Cdk4 client protein: A mechanism of chaperoning kinase clients by exploiting weak spots of intrinsically dynamic kinase domains.PLoS One. 2017 Dec 21;12(12):e0190267. doi: 10.1371/journal.pone.0190267. eCollection 2017. PLoS One. 2017. PMID: 29267381 Free PMC article.
-
Targeting Hsp90-Cdc37: A Promising Therapeutic Strategy by Inhibiting Hsp90 Chaperone Function.Curr Drug Targets. 2017;18(13):1572-1585. doi: 10.2174/1389450117666160527125522. Curr Drug Targets. 2017. PMID: 27231111 Review.
Cited by
-
Natural Withanolides in the Treatment of Chronic Diseases.Adv Exp Med Biol. 2016;928:329-373. doi: 10.1007/978-3-319-41334-1_14. Adv Exp Med Biol. 2016. PMID: 27671823 Free PMC article. Review.
-
In Silico Analysis of Microarray-Based Gene Expression Profiles Predicts Tumor Cell Response to Withanolides.Microarrays (Basel). 2012 May 22;1(1):44-63. doi: 10.3390/microarrays1010044. Microarrays (Basel). 2012. PMID: 27605335 Free PMC article.
-
Targeting the Hsp90-Cdc37-client protein interaction to disrupt Hsp90 chaperone machinery.J Hematol Oncol. 2018 Apr 27;11(1):59. doi: 10.1186/s13045-018-0602-8. J Hematol Oncol. 2018. PMID: 29699578 Free PMC article. Review.
-
Blocking protein kinase C signaling pathway: mechanistic insights into the anti-leishmanial activity of prospective herbal drugs from Withania somnifera.BMC Genomics. 2012;13 Suppl 7(Suppl 7):S20. doi: 10.1186/1471-2164-13-S7-S20. Epub 2012 Dec 13. BMC Genomics. 2012. PMID: 23281834 Free PMC article.
-
Withanolides-induced breast cancer cell death is correlated with their ability to inhibit heat protein 90.PLoS One. 2012;7(5):e37764. doi: 10.1371/journal.pone.0037764. Epub 2012 May 31. PLoS One. 2012. PMID: 22701533 Free PMC article.
References
-
- Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ. A high-affinity conformation of Hsp90 confers tumor selectivity on Hsp90 inhibitors. Clin Cancer Res. 2003;9(16):6126s–6126s. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

