[A randomized study on the effects of paclitaxel liposme and cisplatin induction chemotherapy followed concurrent chemoradiotherapy and sequential radiotherapy on locally advanced non-small cell lung cancer patients]
- PMID: 21342644
- PMCID: PMC5999775
- DOI: 10.3779/j.issn.1009-3419.2011.02.06
[A randomized study on the effects of paclitaxel liposme and cisplatin induction chemotherapy followed concurrent chemoradiotherapy and sequential radiotherapy on locally advanced non-small cell lung cancer patients]
Abstract
Background and objective: Sequential and concurrent chemoradiotherapy were widely studied in locally advanced non-small cell lung cancer (NSCLC), but the reports of induction chemotherapy followed concurrent chemoradiotherapy are rare so far. The little side effects of paclitaxel liposme may be convenient to carry out induction chemotherapy followed concurrent chemoradiotherapy. The aim of this study is to compare the effects and side effects of TP regimen (Paclitaxel liposme and cisplatin) induction chemotherapy followed concurrent chemoradiotherapy with sequential radiotherapy on locally advanced NSCLC.
Methods: Sixty locally advanced NSCLC patients were randomly divided into group A, induction chemotherapy followed concurrent chemoradiotherapy and group B, sequential radiotherapy group. The patients in group A received 2-3 cycles of induced chemotherapy included of Paclitaxel liposme 135 mg/m²-175 mg/m², d1 and cisplatin 70 mg/m²-80 mg/m², d2, 3 weeks repeat and after 2-3 cycles followed by concurrent chemoradiotherapy. The patients in group B received chemotherapy, (as described above in group A) 4-6 cycles of chemotherapy followed one cycle of radiotherapy. The three-dimensional conformal radiotherapy at the total dose of 56 Gy-70 Gy was applied in all patients.
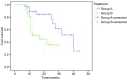
Results: The response rate in group A and group B were 80.3% and 60%, respectively (P=0.042). 1-year survival rates were 71.4% and 53.2%, respectively (P=0.18). And there were no significant difference of myelosuppression, radiation esophagitis and pulmonary fibrosis between the two groups (P=0.09, P=0.147, P=0.276, respectively).
Conclusions: The recent effects of induction chemotherapy followed by concurrent chemoradiotherapy group were better than sequential radiotherapy group on locally advanced NSCLC and there was no significant difference in side effects between the two groups.
背景与目的: 序贯放化疗及同期放化疗在局部晚期肺癌治疗中得以广泛研究,而诱导加同期放化疗的研究尚少。紫杉醇脂质体副作用少,可使诱导加同期放化疗更顺利地实施。本文旨在比较紫杉醇脂质体加顺铂(TP方案)诱导加同期放化疗和序贯放化疗治疗局部晚期非小细胞肺癌(non-small cell lung cancer, NSCLC)的疗效及毒副作用。
方法: 我院60例局部晚期NSCLC患者随机分为诱导加同期放化疗组(A组)和序贯放化疗组(B组)。A组:诱导化疗2个-3个周期后行同期放化疗,放疗的第1天及第22天予TP方案化疗(紫杉醇脂质体135 mg/m2-175 mg/m2,d1;顺铂70 mg/m2-80 mg/m2,d2),期间持续放疗。B组:化疗方案同前,化疗4个-6个周期后,行放疗。两组放疗方式均为三维适形放疗,总剂量为56 Gy-70 Gy。观察和比较两组的疗效和毒副作用。
结果: A组、B组总有效率分别为80.3%和60%,组间有统计学差异(P=0.042);1年生存率分别为71.4%和53.2%,组间无统计学差异(P=0.18);骨髓抑制发生率分别为90%和73.3%,组间无统计学差异(P=0.09);放射性食管炎发生率分别为50%和36.7%,组间无统计学差异(P=0.147);肺纤维化发生率分别为30%和20%,组间无统计学差异(P=0.276)。
结论: 在晚期NSCLC的局部治疗中,TP方案诱导加同期放化疗较序贯放化疗的近期疗效好,但毒副反应无明显区别。
Figures
Similar articles
-
[Initial outcome of induction chemotherapy with weekly paclitaxel followed by three-dimensional conformal radiotherapy and concurrent weekly paclitaxel for stage III non-small cell lung cancer].Ai Zheng. 2006 Oct;25(10):1279-83. Ai Zheng. 2006. PMID: 17059776 Chinese.
-
Induction paclitaxel and carboplatin followed by concurrent chemoradiotherapy in patients with unresectable, locally advanced non-small cell lung carcinoma: report of Fox Chase Cancer Center study 94-001.Semin Oncol. 1997 Aug;24(4 Suppl 12):S12-89-S12-95. Semin Oncol. 1997. PMID: 9331129 Clinical Trial.
-
[Weekly paclitaxel and carboplatin with concurrent three dimensional conformal radiotherapy for locally advanced non small cell lung cancer].Zhonghua Zhong Liu Za Zhi. 2007 Oct;29(10):769-72. Zhonghua Zhong Liu Za Zhi. 2007. PMID: 18396691 Chinese.
-
Radiotherapy and chemotherapy in locally advanced non-small cell lung cancer: preclinical and early clinical data.Hematol Oncol Clin North Am. 2004 Feb;18(1):41-53. doi: 10.1016/s0889-8588(03)00138-2. Hematol Oncol Clin North Am. 2004. PMID: 15005280 Review.
-
Sequential chemo- and radiochemotherapy with weekly paclitaxel (Taxol) and 3D-conformal radiotherapy of stage III inoperable non-small cell lung cancer. Results of a dose escalation study.Lung Cancer. 2001 May;32(2):163-71. doi: 10.1016/s0169-5002(00)00216-6. Lung Cancer. 2001. PMID: 11325487 Review.
Cited by
-
[A randomized trial of liposomal paclitaxel plus cisplatin as first-line therapy for advanced non-small cell lung cancer].Zhongguo Fei Ai Za Zhi. 2012 Apr;15(4):208-12. doi: 10.3779/j.issn.1009-3419.2012.04.03. Zhongguo Fei Ai Za Zhi. 2012. PMID: 22510505 Free PMC article. Clinical Trial. Chinese.
References
-
- Jia JW, Wu YM, Guo SL, et al. Comparison of paclitaxel liposome and traditional taxol plus cisplatin in elderly patients with non-small cell lung cancer. J Fourth Mil Med Univer. 2008;29(17):1604–1606. doi: 10.3321/j.issn:1000-2790.2008.17.020. - DOI
- 贾 晋伟, 吴 亚梅, 郭 述良, et al. 紫杉醇脂质体与传统紫杉醇联合顺铂治疗老年非小细胞肺癌对比研究. 第四军医大学学报. 2008;29(17):1604–1606. doi: 10.3321/j.issn:1000-2790.2008.17.020. - DOI
-
- Wang AL, Zhou H, Wen XP, et al. Therapeutic efficacy of chemotherapy combined with radiotherapy in 527 patients with stage Ⅲ and Ⅳ non-small cell lung cancer. http://www.cqvip.com/Main/Detail.aspx?id=1000099314 Chin J Lung Cancer. 2007;10(3):219–222. - PubMed
- 汪 安兰, 周 辉, 文 晓萍, et al. 527例不能手术的Ⅲ/Ⅳ期非小细胞肺癌化放综合治疗的疗效分析. http://www.cqvip.com/Main/Detail.aspx?id=1000099314 中国肺癌杂志. 2007;10(3):219–222.
-
- Wu YL, Jiang GL, Liao ML, et al. Consensus on chemoradiotherapy for local advanced NSCLC. http://www.cqvip.com/QK/87137X/2005003/16065755.html J Evidence-Based Med. 2005;5(3):186–188.
- 吴 一龙, 蒋 国梁, 廖 美琳, et al. 局部晚期非小细胞肺癌化放疗共识. http://www.cqvip.com/QK/87137X/2005003/16065755.html 循证医学. 2005;5(3):186–188.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical