Label-free multiplexed virus detection using spectral reflectance imaging
- PMID: 21342761
- PMCID: PMC3065545
- DOI: 10.1016/j.bios.2011.01.019
Label-free multiplexed virus detection using spectral reflectance imaging
Abstract
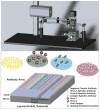
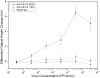
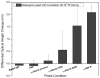
We demonstrate detection of whole viruses and viral proteins with a new label-free platform based on spectral reflectance imaging. The Interferometric Reflectance Imaging Sensor (IRIS) has been shown to be capable of sensitive protein and DNA detection in a real time and high-throughput format. Vesicular stomatitis virus (VSV) was used as the target for detection as it is well-characterized for protein composition and can be modified to express viral coat proteins from other dangerous, highly pathogenic agents for surrogate detection while remaining a biosafety level 2 agent. We demonstrate specific detection of intact VSV virions achieved with surface-immobilized antibodies acting as capture probes which is confirmed using fluorescence imaging. The limit of detection is confirmed down to 3.5 × 10(5)plaque-forming units/mL (PFUs/mL). To increase specificity in a clinical scenario, both the external glycoprotein and internal viral proteins were simultaneously detected with the same antibody arrays with detergent-disrupted purified VSV and infected cell lysate solutions. Our results show sensitive and specific virus detection with a simple surface chemistry and minimal sample preparation on a quantitative label-free interferometric platform.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures



References
-
- Stamboulian D, Bonvehi PE, Nacinovich FM, Cox N. Infect Dis Clin North Am. 2000;14 (1):141–166. - PubMed
-
- Amano Y, Cheng Q. Anal Bioanal Chem. 2005;381 (1):156–164. - PubMed
-
- Charlton B, Crossley B, Hietala S. Comp Immunol Microbiol Infect Dis. 2009;32 (4):341–350. - PubMed
-
- Baner J, Gyarmati P, Yacoub A, Hakhverdyan M, Stenberg J, Ericsson O, Nilsson M, Landegren U, Belak S. J Virol Methods. 2007;143 (2):200–206. - PubMed
-
- Baxi MK, Baxi S, Clavijo A, Burton KM, Deregt D. Vet J. 2006;172 (3):473–481. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

