An Ad5-vectored HIV-1 vaccine elicits cell-mediated immunity but does not affect disease progression in HIV-1-infected male subjects: results from a randomized placebo-controlled trial (the Step study)
- PMID: 21343146
- PMCID: PMC3119328
- DOI: 10.1093/infdis/jiq114
An Ad5-vectored HIV-1 vaccine elicits cell-mediated immunity but does not affect disease progression in HIV-1-infected male subjects: results from a randomized placebo-controlled trial (the Step study)
Abstract
Background: The Step study was a randomized trial to determine whether an adenovirus type 5 (Ad5) vector vaccine, which elicits T cell immunity, can lead to control of human immunodeficiency virus (HIV) replication in participants who became HIV-infected after vaccination.
Methods: We evaluated the effect of the vaccine on trends in HIV viral load, CD4+ T cell counts, time to initiation of antiretroviral therapy (ART), and AIDS-free survival in 87 male participants who became infected with HIV during the Step study and who had a median of 24 months of post-infection follow-up.
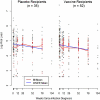
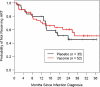
Results: There was no overall effect of vaccine on mean log(10) viral load (estimated difference between groups, -0.11; P = .47). In a subset of subjects with protective HLA types (B27, B57, B58), mean HIV-1 RNA level over time was lower among vaccine recipients. There was no significant difference in CD4+ T cell counts, time to ART initiation, or in AIDS-free survival between HIV-1-infected subjects who received vaccine versus those who received placebo.
Conclusions: HIV RNA levels, CD4+ T cell counts, time to initiation of ART, and AIDS-free survival were similar in vaccine and placebo recipients. There may have been a favorable effect of vaccine on HIV-1 RNA levels in participants with HLA types associated with better control of HIV-1.
Trial registration: ClinicalTrials.gov NCT00095576.
Figures

Comment in
-
The STEP study provides a hint that vaccine induction of the right CD8+ T cell responses can facilitate immune control of HIV.J Infect Dis. 2011 Mar 15;203(6):753-5. doi: 10.1093/infdis/jiq119. J Infect Dis. 2011. PMID: 21343145 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 AI069438/AI/NIAID NIH HHS/United States
- U01 AI069486/AI/NIAID NIH HHS/United States
- U01 AI069420/AI/NIAID NIH HHS/United States
- U01 AI069409/AI/NIAID NIH HHS/United States
- UM1 AI069415/AI/NIAID NIH HHS/United States
- UM1 AI069470/AI/NIAID NIH HHS/United States
- UM1 AI068614/AI/NIAID NIH HHS/United States
- U01 AI069421/AI/NIAID NIH HHS/United States
- U01 AI069415/AI/NIAID NIH HHS/United States
- U01 AI069554/AI/NIAID NIH HHS/United States
- U01 AI068618/AI/NIAID NIH HHS/United States
- U01 AI069412/AI/NIAID NIH HHS/United States
- U01 AI069496/AI/NIAID NIH HHS/United States
- UM1 AI069438/AI/NIAID NIH HHS/United States
- U01 AI069470/AI/NIAID NIH HHS/United States
- U01 AI068635/AI/NIAID NIH HHS/United States
- UM1 AI069496/AI/NIAID NIH HHS/United States
- U01 AI069511/AI/NIAID NIH HHS/United States
- U01 AI048021/AI/NIAID NIH HHS/United States
- UM1 AI069511/AI/NIAID NIH HHS/United States
- U01 AI069452/AI/NIAID NIH HHS/United States
- U01 AI068614/AI/NIAID NIH HHS/United States
- U01 AI069414/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

