The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4
- PMID: 21343298
- PMCID: PMC3075677
- DOI: 10.1074/jbc.M110.192138
The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4
Abstract
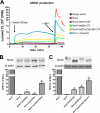
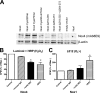
In contrast to the NADPH oxidases Nox1 and Nox2, which generate superoxide (O(2)(·-)), Nox4 produces hydrogen peroxide (H(2)O(2)). We constructed chimeric proteins and mutants to address the protein region that specifies which reactive oxygen species is produced. Reactive oxygen species were measured with luminol/horseradish peroxidase and Amplex Red for H(2)O(2) versus L-012 and cytochrome c for O(2)(·-). The third extracytosolic loop (E-loop) of Nox4 is 28 amino acids longer than that of Nox1 or Nox2. Deletion of E-loop amino acids only present in Nox4 or exchange of the two cysteines in these stretches switched Nox4 from H(2)O(2) to O(2)(·-) generation while preserving expression and intracellular localization. In the presence of an NO donor, the O(2)()-producing Nox4 mutants, but not wild-type Nox4, generated peroxynitrite, excluding artifacts of the detection system as the apparent origin of O(2)(·-). In Cos7 cells, in which Nox4 partially localizes to the plasma membrane, an antibody directed against the E-loop decreased H(2)O(2) but increased O(2)(·-) formation by Nox4 without affecting Nox1-dependent O(2)(·-) formation. The E-loop of Nox4 but not Nox1 and Nox2 contains a highly conserved histidine that could serve as a source for protons to accelerate spontaneous dismutation of superoxide to form H(2)O(2). Mutation of this but not of four other conserved histidines also switched Nox4 from H(2)O(2) to O(2)(·-) formation. Thus, H(2)O(2) formation is an intrinsic property of Nox4 that involves its E-loop. The structure of the E-loop may hinder O(2)(·-) egress and/or provide a source for protons, allowing dismutation to form H(2)O(2).
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

