Survival strategies of yeast and filamentous fungi against the antifungal protein AFP
- PMID: 21343301
- PMCID: PMC3077587
- DOI: 10.1074/jbc.M110.203588
Survival strategies of yeast and filamentous fungi against the antifungal protein AFP
Abstract
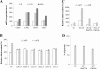
The activities of signaling pathways are critical for fungi to survive antifungal attack and to maintain cell integrity. However, little is known about how fungi respond to antifungals, particularly if these interact with multiple cellular targets. The antifungal protein AFP is a very potent inhibitor of chitin synthesis and membrane integrity in filamentous fungi and has so far not been reported to interfere with the viability of yeast strains. With the hypothesis that the susceptibility of fungi toward AFP is not merely dependent on the presence of an AFP-specific target at the cell surface but relies also on the cell's capacity to counteract AFP, we used a genetic approach to decipher defense strategies of the naturally AFP-resistant strain Saccharomyces cerevisiae. The screening of selected strains from the yeast genomic deletion collection for AFP-sensitive phenotypes revealed that a concerted action of calcium signaling, TOR signaling, cAMP-protein kinase A signaling, and cell wall integrity signaling is likely to safeguard S. cerevisiae against AFP. Our studies uncovered that the yeast cell wall gets fortified with chitin to defend against AFP and that this response is largely dependent on calcium/Crz1p signaling. Most importantly, we observed that stimulation of chitin synthesis is characteristic for AFP-resistant fungi but not for AFP-sensitive fungi, suggesting that this response is a successful strategy to protect against AFP. We finally propose the adoption of the damage-response framework of microbial pathogenesis for the interactions of antimicrobial proteins and microorganisms in order to comprehensively understand the outcome of an antifungal attack.
Figures
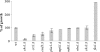






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

