Thymopoietic and bone marrow response to murine Pneumocystis pneumonia
- PMID: 21343353
- PMCID: PMC3088129
- DOI: 10.1128/IAI.01213-10
Thymopoietic and bone marrow response to murine Pneumocystis pneumonia
Abstract
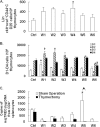
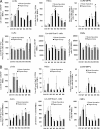
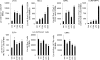
CD4(+) T cells play a key role in host defense against Pneumocystis infection. To define the role of naïve CD4(+) T cell production through the thymopoietic response in host defense against Pneumocystis infection, Pneumocystis murina infection in the lung was induced in adult male C57BL/6 mice with and without prior thymectomy. Pneumocystis infection caused a significant increase in the number of CCR9(+) multipotent progenitor (MPP) cells in the bone marrow and peripheral circulation, an increase in populations of earliest thymic progenitors (ETPs) and double negative (DN) thymocytes in the thymus, and recruitment of naïve and total CD4(+) T cells into the alveolar space. The level of murine signal joint T cell receptor excision circles (msjTRECs) in spleen CD4(+) cells was increased at 5 weeks post-Pneumocystis infection. In thymectomized mice, the numbers of naïve, central memory, and total CD4(+) T cells in all tissues examined were markedly reduced following Pneumocystis infection. This deficiency of naïve and central memory CD4(+) T cells was associated with delayed pulmonary clearance of Pneumocystis. Extracts of Pneumocystis resulted in an increase in the number of CCR9(+) MPPs in the cultured bone marrow cells. Stimulation of cultured bone marrow cells with ligands to Toll-like receptor 2 ([TLR-2] zymosan) and TLR-9 (ODN M362) each caused a similar increase in CCR9(+) MPP cells via activation of the Jun N-terminal protein kinase (JNK) pathway. These results demonstrate that enhanced production of naïve CD4(+) T lymphocytes through the thymopoietic response and enhanced delivery of lymphopoietic precursors from the bone marrow play an important role in host defense against Pneumocystis infection.
Figures
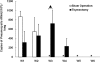



References
-
- Akashi K., Traver D., Miyamoto T., Weissman I. L. 2000. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404:193–197 - PubMed
-
- Akashi K. 2007. Cartography of hematopoietic stem cell commitment dependent upon a reporter for transcription factor activation. Ann. N. Y. Acad. Sci. 1106:76–81 - PubMed
-
- Allman D., et al. 2003. Thymopoiesis independent of common lymphoid progenitors. Nat. Immunol. 4:168–174 - PubMed
-
- Beq S., et al. 2006. IL-7 induces immunological improvement in SIV-infected rhesus macaques under antiviral therapy. J. Immunol. 176:914–922 - PubMed
-
- Choyke P. L., et al. 1987. Thymic atrophy and regrowth in response to chemotherapy: CT evaluation. AJR Am. J. Roentgenol. 149:269–272 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

