Epithelial phosphatidylinositol-3-kinase signaling is required for β-catenin activation and host defense against Citrobacter rodentium infection
- PMID: 21343355
- PMCID: PMC3088123
- DOI: 10.1128/IAI.01025-10
Epithelial phosphatidylinositol-3-kinase signaling is required for β-catenin activation and host defense against Citrobacter rodentium infection
Abstract
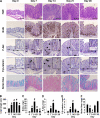
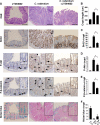
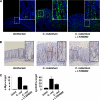
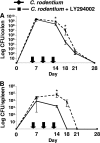
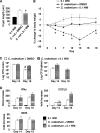
Citrobacter rodentium infection of mice induces cell-mediated immune responses associated with crypt hyperplasia and epithelial β-catenin signaling. Recent data suggest that phosphatidylinositol-3-kinase (PI3K)/Akt signaling cooperates with Wnt to activate β-catenin in intestinal stem and progenitor cells through phosphorylation at Ser552 (P-β-catenin(552)). Our aim was to determine whether epithelial PI3K/Akt activation is required for β-catenin signaling and host defense against C. rodentium. C57BL/6 mice were infected with C. rodentium and treated with dimethyl sulfoxide (DMSO) (vehicle control) or with the PI3K inhibitor LY294002 or wortmannin. The effects of infection on PI3K activation and β-catenin signaling were analyzed by immunohistochemistry. The effects of PI3K inhibition on host defense were analyzed by the quantification of splenic and colon bacterial clearance, and adaptive immune responses were measured by real-time PCR (RT-PCR) and enzyme-linked immunosorbent assay (ELISA). Increased numbers of P-β-catenin(552)-stained epithelial cells were found throughout expanded crypts in C. rodentium colitis. We show that the inhibition of PI3K signaling attenuates epithelial Akt activation, the Ser552 phosphorylation and activation of β-catenin, and epithelial cell proliferative responses during C. rodentium infection. PI3K inhibition impairs bacterial clearance despite having no impact on mucosal cytokine (gamma interferon [IFN-γ], tumor necrosis factor [TNF], interleukin-17 [IL-17], and IL-1β) or chemokine (CXCL1, CXCL5, CXCL9, and CXCL10) induction. The results suggest that the host defense against C. rodentium requires epithelial PI3K activation to induce Akt-mediated β-catenin signaling and the clearance of C. rodentium independent of adaptive immune responses.
Figures

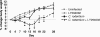



References
-
- Aujla S. J., Kolls J. K. 2009. IL-22: a critical mediator in mucosal host defense. J. Mol. Med. 87:451–454 - PubMed
-
- Bader A. G., Kang S., Zhao L., Vogt P. K. 2005. Oncogenic PI3K deregulates transcription and translation. Nat. Rev. Cancer 5:921–929 - PubMed
-
- Bjerknes M., Cheng H. 1999. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology 116:7–14 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

