The cellular protein La functions in enhancement of virus release through lipid rafts facilitated by murine leukemia virus glycosylated Gag
- PMID: 21343359
- PMCID: PMC3042739
- DOI: 10.1128/mBio.00341-10
The cellular protein La functions in enhancement of virus release through lipid rafts facilitated by murine leukemia virus glycosylated Gag
Abstract
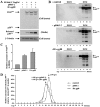
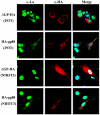
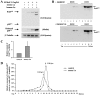
Murine leukemia viruses (MuLVs) encode two forms of Gag polyprotein: the precursor for the viral core proteins (Pr65(gag) for Moloney MuLV [M-MuLV]) and a longer glycosylated form (glyco-gag, or gPr80(gag)). gPr80(gag) is translated from the same unspliced viral RNA as Pr65(gag), from an upstream in-frame CUG initiation codon. As a result, gPr80(gag) contains 88 unique N-terminal amino acids that include a signal peptide that conducts gPr80(gag) into the rough endoplasmic reticulum, where it is glycosylated, exported to the cell surface, and cleaved into two proteins of 55 and 40 kDa. The amino-terminal 55-kDa protein remains cell associated with the 88 unique amino acids exposed to the cytosol. We previously showed that gPr80(gag) facilitates efficient M-MuLV release through lipid rafts. In this report, we found that the unique N-terminal domain of gPr80(gag) is sufficient to facilitate enhanced M-MuLV particle release from transfected 293T cells. A search for cellular proteins involved in gPr80(gag) function led to cellular La protein. Overexpression of mouse or human La enhanced M-MuLV particle release in the absence of glyco-gag, and the released virus had a reduced buoyant density characteristic of increased cholesterol content. Moreover, small interfering RNA (siRNA) knockdown of human La abolished glyco-gag enhancement of M-MuLV release. These results implicate La as a cellular protein involved in M-MuLV glyco-gag function. We also found that overexpression of mouse or human La could enhance HIV-1 release in the absence of gPr80(gag). Therefore, M-MuLV and HIV-1 may share a pathway for release through lipid rafts involving La.
Copyright © 2011 Nitta et al.
Figures





References
-
- Prats A. C., De Billy G., Wang P., Darlix J. L. 1989. CUG initiation codon used for the synthesis of a cell surface antigen coded by the murine leukemia virus. J. Mol. Biol. 205:363–372 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
