synMuv B proteins antagonize germline fate in the intestine and ensure C. elegans survival
- PMID: 21343362
- PMCID: PMC3042865
- DOI: 10.1242/dev.059501
synMuv B proteins antagonize germline fate in the intestine and ensure C. elegans survival
Abstract
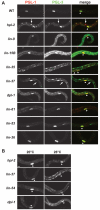
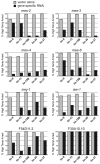
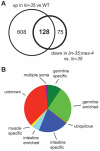
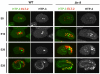
Previous studies demonstrated that a subset of synMuv B mutants ectopically misexpress germline-specific P-granule proteins in their somatic cells, suggesting a failure to properly orchestrate a soma/germline fate decision. Surprisingly, this fate confusion does not affect viability at low to ambient temperatures. Here, we show that, when grown at high temperature, a majority of synMuv B mutants irreversibly arrest at the L1 stage. High temperature arrest (HTA) is accompanied by upregulation of many genes characteristic of germ line, including genes encoding components of the synaptonemal complex and other meiosis proteins. HTA is suppressed by loss of global regulators of germline chromatin, including MES-4, MRG-1, ISW-1 and the MES-2/3/6 complex, revealing that arrest is caused by somatic cells possessing a germline-like chromatin state. Germline genes are preferentially misregulated in the intestine, and necessity and sufficiency tests demonstrate that the intestine is the tissue responsible for HTA. We propose that synMuv B mutants fail to erase or antagonize an inherited germline chromatin state in somatic cells during embryonic and early larval development. As a consequence, somatic cells gain a germline program of gene expression in addition to their somatic program, leading to a mixed fate. Somatic expression of germline genes is enhanced at elevated temperature, leading to developmentally compromised somatic cells and arrest of newly hatched larvae.
Figures

References
-
- Altun Z. F., Hall D. H. (2009). Alimentary system, intestine. In WormAtlas, doi:10.3908/wormatlas.1.4
-
- Beitel G. J., Lambie E. J., Horvitz H. R. (2000). The C. elegans gene lin-9,which acts in an Rb-related pathway, is required for gonadal sheath cell development and encodes a novel protein. Gene 254, 253-263 - PubMed
-
- Bender A. M., Wells O., Fay D. S. (2004). lin-35/Rb and xnp-1/ATR-X function redundantly to control somatic gonad development in C. elegans. Dev. Biol. 273, 335-349 - PubMed
-
- Bender L. B., Cao R., Zhang Y., Strome S. (2004). The MES-2/MES-3/MES-6 complex and regulation of histone H3 methylation in C. elegans. Curr. Biol. 14, 1639-1643 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

