Effect of life history on microRNA expression during C. elegans development
- PMID: 21343388
- PMCID: PMC3062175
- DOI: 10.1261/rna.2310111
Effect of life history on microRNA expression during C. elegans development
Abstract
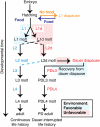
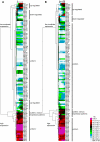
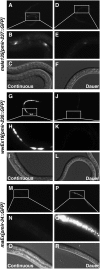
Animals have evolved mechanisms to ensure the robustness of developmental outcomes to changing environments. MicroRNA expression may contribute to developmental robustness because microRNAs are key post-transcriptional regulators of developmental gene expression and can affect the expression of multiple target genes. Caenorhabditis elegans provides an excellent model to study developmental responses to environmental conditions. In favorable environments, C. elegans larvae develop rapidly and continuously through four larval stages. In contrast, in unfavorable conditions, larval development may be interrupted at either of two diapause stages: The L1 diapause occurs when embryos hatch in the absence of food, and the dauer diapause occurs after the second larval stage in response to environmental stimuli encountered during the first two larval stages. Dauer larvae are stress resistant and long lived, permitting survival in harsh conditions. When environmental conditions improve, dauer larvae re-enter development, and progress through two post-dauer larval stages to adulthood. Strikingly, all of these life history options (whether continuous or interrupted) involve an identical pattern and sequence of cell division and cell fates. To identify microRNAs with potential functions in buffering development in the context of C. elegans life history options, we used multiplex real-time PCR to assess the expression of 107 microRNAs throughout development in both continuous and interrupted life histories. We identified 17 microRNAs whose developmental profile of expression is affected by dauer life history and/or L1 diapause, compared to continuous development. Hence these microRNAs could function to regulate gene expression programs appropriate for different life history options in the developing worm.
Figures

References
-
- AppliedBiosystems 2008. Guide to performing relative quantitation of gene expression using real-time quantitative PCR. http://www3.appliedbiosystems.com/cms/groups/mcb_support/documents/gener...
-
- Barriere A, Felix MA 2005. High local genetic diversity and low outcrossing rate in Caenorhabditis elegans natural populations. Curr Biol 15: 1176–1184 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
