Differential drug efflux or accumulation does not explain variation in the chloroquine response of Plasmodium falciparum strains expressing the same isoform of mutant PfCRT
- PMID: 21343459
- PMCID: PMC3088201
- DOI: 10.1128/AAC.01167-10
Differential drug efflux or accumulation does not explain variation in the chloroquine response of Plasmodium falciparum strains expressing the same isoform of mutant PfCRT
Abstract
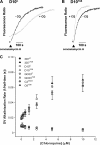
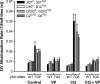
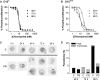
Mutant forms of the Plasmodium falciparum chloroquine resistance transporter (PfCRT) mediate chloroquine resistance by effluxing the drug from the parasite's digestive vacuole, the acidic organelle in which chloroquine exerts its parasiticidal effect. However, different parasites bearing the same mutant form of PfCRT can vary substantially in their chloroquine susceptibility. Here, we have investigated the biochemical basis for the difference in chloroquine response among transfectant parasite lines having different genetic backgrounds but bearing the same mutant form of PfCRT. Despite showing significant differences in their chloroquine susceptibility, all lines with the mutant PfCRT showed a similar chloroquine-induced H+ leak from the digestive vacuole, indicative of similar rates of PfCRT-mediated chloroquine efflux. Furthermore, all lines showed similarly reduced levels of drug accumulation. Factors other than chloroquine efflux and accumulation therefore influence the susceptibility to this drug in parasites expressing mutant PfCRT. Furthermore, in some but not all strains bearing mutant PfCRT, the 50% inhibitory concentration (IC50) for chloroquine and the degree of resistance compared to that of recombinant control parasites varied with the length of the parasite growth assays. In these parasites, the 50% inhibitory concentration for chloroquine measured in 72- or 96-h assays was significantly lower than that measured in 48-h assays. This highlights the importance of considering the first- and second-cycle activities of chloroquine in future studies of parasite susceptibility to this drug.
Figures


References
-
- Abu Bakar N., Klonis N., Hanssen E., Chan C., Tilley L. 2010. Digestive-vacuole genesis and endocytic processes in the early intraerythrocytic stages of Plasmodium falciparum. J. Cell Sci. 123:441–450 - PubMed
-
- Allen R. J., Kirk K. 2010. Plasmodium falciparum culture: the benefits of shaking. Mol. Biochem. Parasitol. 169:63–65 - PubMed
-
- Becker K., et al. 2004. Oxidative stress in malaria parasite-infected erythrocytes: host-parasite interactions. Int. J. Parasitol. 34:163–189 - PubMed
-
- Bray P. G., et al. 2005. Defining the role of PfCRT in Plasmodium falciparum chloroquine resistance. Mol. Microbiol. 56:323–333 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

