Prdm16 is a physiologic regulator of hematopoietic stem cells
- PMID: 21343612
- PMCID: PMC3109532
- DOI: 10.1182/blood-2010-08-300145
Prdm16 is a physiologic regulator of hematopoietic stem cells
Abstract
Fetal liver and adult bone marrow hematopoietic stem cells (HSCs) renew or differentiate into committed progenitors to generate all blood cells. PRDM16 is involved in human leukemic translocations and is expressed highly in some karyotypically normal acute myeloblastic leukemias. As many genes involved in leukemogenic fusions play a role in normal hematopoiesis, we analyzed the role of Prdm16 in the biology of HSCs using Prdm16-deficient mice. We show here that, within the hematopoietic system, Prdm16 is expressed very selectively in the earliest stem and progenitor compartments, and, consistent with this expression pattern, is critical for the establishment and maintenance of the HSC pool during development and after transplantation. Prdm16 deletion enhances apoptosis and cycling of HSCs. Expression analysis revealed that Prdm16 regulates a remarkable number of genes that, based on knockout models, both enhance and suppress HSC function, and affect quiescence, cell cycling, renewal, differentiation, and apoptosis to various extents. These data suggest that Prdm16 may be a critical node in a network that contains negative and positive feedback loops and integrates HSC renewal, quiescence, apoptosis, and differentiation.
Figures

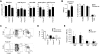

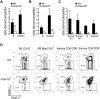
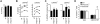

Comment in
-
Joining the stem cell maintenance squad.Blood. 2011 May 12;117(19):5011-2. doi: 10.1182/blood-2011-03-340356. Blood. 2011. PMID: 21566097 No abstract available.
References
-
- Ernst P, Fisher JK, Avery W, et al. Definitive hematopoiesis requires the mixed-lineage leukemia gene. Dev Cell. 2004;6(3):437–443. - PubMed
-
- McMahon KA, Hiew SY, Hadjur S, et al. Mll has a critical role in fetal and adult hematopoietic stem cell self-renewal. Cell Stem Cell. 2007;1(3):338–345. - PubMed
-
- Goyama S, Yamamoto G, Shimabe M, et al. Evi-1 is a critical regulator for hematopoietic stem cells and transformed leukemic cells. Cell Stem Cell. 2008;3(2):207–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

