Emerging role for ERM proteins in cell adhesion and migration
- PMID: 21343695
- PMCID: PMC3084986
- DOI: 10.4161/cam.5.2.15081
Emerging role for ERM proteins in cell adhesion and migration
Abstract
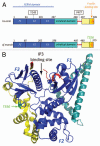
The highly related ERM (Ezrin, Radixin, Moesin) proteins provide a regulated linkage between the membrane and the underlying actin cytoskeleton. They also provide a platform for the transmission of signals in responses to extracellular cues. Studies in different model organisms and in cultured cells have highlighted the importance of ERM proteins in the generation and maintenance of specific domains of the plasma membrane. A central question is how do ERM proteins coordinate actin filament organization and membrane protein transport/stability with signal transduction pathways to build up complex structures? Through their interaction with numerous partners including membrane proteins, actin cytoskeleton and signaling molecules, ERM proteins have the ability to organize multiprotein complexes in specific cellular compartments. Likewise, ERM proteins participate in diverse functions including cell morphogenesis, endocytosis/exocytosis, adhesion and migration. This review focuses on aspects still poorly understood related to the function of ERM proteins in epithelial cell adhesion and migration.
Figures
References
-
- Sato N, Funayama N, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. A gene family consisting of ezrin, radixin and moesin. Its specific localization at actin filament/plasma membrane association sites. J Cell Sci. 1992;103:131–143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
