Suppression of inflammation-associated factors by indole-3-carbinol in mice fed high-fat diets and in isolated, co-cultured macrophages and adipocytes
- PMID: 21343904
- PMCID: PMC3238050
- DOI: 10.1038/ijo.2011.12
Suppression of inflammation-associated factors by indole-3-carbinol in mice fed high-fat diets and in isolated, co-cultured macrophages and adipocytes
Erratum in
- Int J Obes (Lond). 2013 Feb;37(2):324
Abstract
Aims: This study investigated the effects of indole-3-carbinol (I3C), a compound from cruciferous vegetables, on various parameters related to obesity, in particular, the parameters of infiltration by macrophages and of inflammatory cytokines expressed during the co-culture of adipocytes and macrophages.
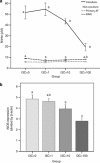
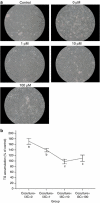
Methods: Male C57BL/6 mice were fed with a control diet (C group), high-fat diet (HF group) and HF+5 mg kg(-1) I3C (HFI group). The I3C was intraperitoneally injected (HFI group) for 12 weeks. Epididymal adipose tissue (AT) was collected and stained for F4/80, a marker of macrophages.
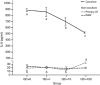
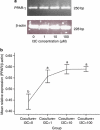
Results: The immunohistochemical staining for F4/80 indicated a greater presence of macrophages in the HF group than in AT from the control and HFI groups. Furthermore, I3C treatment, in an in vitro cell culture system, decreased expression of inducible nitric oxide synthase (iNOS), decreased nitrite content and enhanced expression of peroxisome proliferator-activated receptor (PPAR-γ). Moreover, in vitro cell culture studies revealed that I3C inhibited intracellular lipid accumulation in hypertrophied adipocytes. In macrophage and primary adipocyte co-culture, I3C inhibited expression of interleukin-6 (IL-6).
Conclusions: In vivo treatment with I3C reduced the infiltration of macrophages in AT, and in vitro addition of I3C to co-cultured macrophages and adipocytes reduced nitrite production and IL-6 expression. With cultures of adipocytes only, I3C inhibited accumulation of intracellular lipid, either by disrupting differentiation, or by directly inhibiting triglyceride synthesis.
Figures

References
-
- Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation. 2004;110:1564–1571. - PubMed
-
- Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, et al. Ludwig, a potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–1145. - PubMed
-
- Zeyda M, Stulnig TM. Obesity, inflammation, and insulin resistance--a mini-review. Gerontology. 2009;55:379–386. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

