A peptide-based vector for efficient gene transfer in vitro and in vivo
- PMID: 21343913
- PMCID: PMC3149163
- DOI: 10.1038/mt.2011.10
A peptide-based vector for efficient gene transfer in vitro and in vivo
Abstract
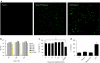
Finding suitable nonviral delivery vehicles for nucleic acid-based therapeutics is a landmark goal in gene therapy. Cell-penetrating peptides (CPPs) are one class of delivery vectors that has been exploited for this purpose. However, since CPPs use endocytosis to enter cells, a large fraction of peptides remain trapped in endosomes. We have previously reported that stearylation of amphipathic CPPs, such as transportan 10 (TP10), dramatically increases transfection of oligonucleotides in vitro partially by promoting endosomal escape. Therefore, we aimed to evaluate whether stearyl-TP10 could be used for the delivery of plasmids as well. Our results demonstrate that stearyl-TP10 forms stable nanoparticles with plasmids that efficiently enter different cell-types in a ubiquitous manner, including primary cells, resulting in significantly higher gene expression levels than when using stearyl-Arg9 or unmodified CPPs. In fact, the transfection efficacy of stearyl-TP10 almost reached the levels of Lipofectamine 2000 (LF2000), however, without any of the observed lipofection-associated toxicities. Most importantly, stearyl-TP10/plasmid nanoparticles are nonimmunogenic, mediate efficient gene delivery in vivo, when administrated intramuscularly (i.m.) or intradermally (i.d.) without any associated toxicity in mice.
Figures


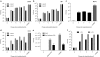


Similar articles
-
Efficient intracellular delivery of nucleic acid pharmaceuticals using cell-penetrating peptides.Acc Chem Res. 2012 Jul 17;45(7):1132-9. doi: 10.1021/ar200256e. Epub 2011 Dec 30. Acc Chem Res. 2012. PMID: 22208383
-
Stearylated antimicrobial peptide [D]-K6L9 with cell penetrating property for efficient gene transfer.Peptides. 2013 Aug;46:33-9. doi: 10.1016/j.peptides.2013.05.011. Epub 2013 May 28. Peptides. 2013. PMID: 23727033
-
Delivery of nucleic acids with a stearylated (RxR)4 peptide using a non-covalent co-incubation strategy.J Control Release. 2010 Jan 4;141(1):42-51. doi: 10.1016/j.jconrel.2009.08.028. Epub 2009 Sep 8. J Control Release. 2010. PMID: 19744531
-
Cell-penetrating peptides for the delivery of nucleic acids.Expert Opin Drug Deliv. 2012 Jul;9(7):823-36. doi: 10.1517/17425247.2012.689285. Epub 2012 May 17. Expert Opin Drug Deliv. 2012. PMID: 22594635 Review.
-
Cell Penetrating Peptide Conjugated Chitosan for Enhanced Delivery of Nucleic Acid.Int J Mol Sci. 2015 Dec 4;16(12):28912-30. doi: 10.3390/ijms161226142. Int J Mol Sci. 2015. PMID: 26690119 Free PMC article. Review.
Cited by
-
Design and characterization of a new peptide vector for short interfering RNA delivery.J Nanobiotechnology. 2015 Jun 9;13:39. doi: 10.1186/s12951-015-0098-0. J Nanobiotechnology. 2015. PMID: 26054932 Free PMC article.
-
Bioreducible polypeptide containing cell-penetrating sequence for efficient gene delivery.Pharm Res. 2013 Aug;30(8):1968-78. doi: 10.1007/s11095-013-1040-5. Epub 2013 Apr 19. Pharm Res. 2013. PMID: 23604924
-
The Formation of Nanoparticles between Small Interfering RNA and Amphipathic Cell-Penetrating Peptides.Mol Ther Nucleic Acids. 2017 Jun 16;7:1-10. doi: 10.1016/j.omtn.2017.02.003. Epub 2017 Feb 10. Mol Ther Nucleic Acids. 2017. PMID: 28624185 Free PMC article.
-
Kinetic Modeling to Accelerate the Development of Nucleic Acid Formulations.ACS Nano. 2021 Oct 26;15(10):16055-16066. doi: 10.1021/acsnano.1c04555. Epub 2021 Oct 12. ACS Nano. 2021. PMID: 34636541 Free PMC article.
-
The Effect of Size and Shape of RNA Nanoparticles on Biodistribution.Mol Ther. 2018 Mar 7;26(3):784-792. doi: 10.1016/j.ymthe.2017.12.018. Epub 2017 Dec 22. Mol Ther. 2018. PMID: 29402549 Free PMC article.
References
-
- Thomas CE, Ehrhardt A., and, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–358. - PubMed
-
- Glover DJ, Lipps HJ., and, Jans DA. Towards safe, non-viral therapeutic gene expression in humans. Nat Rev Genet. 2005;6:299–310. - PubMed
-
- Viola JR, El-Andaloussi S, Oprea II., and, Smith CI. Non-viral nanovectors for gene delivery: factors that govern successful therapeutics. Expert Opin Drug Deliv. 2010;7:721–735. - PubMed
-
- Pack DW, Hoffman AS, Pun S., and, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4:581–593. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

