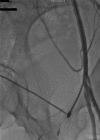
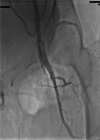
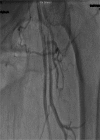
Acute complication due to impella 2.5 device (superficial femoral artery thrombosis): managed successfully with novel aspiration thrombectomy catheter (pronto v3)
- PMID: 21344022
- PMCID: PMC3041237
- DOI: 10.4137/CMC.S6157
Acute complication due to impella 2.5 device (superficial femoral artery thrombosis): managed successfully with novel aspiration thrombectomy catheter (pronto v3)
Abstract
The Impella recover LP 2.5 is a percutaneous left ventricular assist device (LVAD) recently approved for use in patients undergoing high risk percutaneous coronary intervention (PCI) and also in cases of cardiogenic shock. There is limited evidence available in literature about its safety, especially with regards to the incidence of local vascular complications, their management and long-term implications. We report here the first case of a serious local vascular complication-superficial femoral artery thrombus formation during Impella recover LP 2.5 use in a high risk PCI which was managed successfully with novel aspiration thrombectomy catheter (Pronto V3), which in itself is the first reported use of Pronto V3 in such a vascular complication.
Keywords: complication of impella; high risk PCI; impella LP 2.5; pronto V3.
Figures
References
-
- Valgimigli M, Steendijk P, Sianos G, Onderwater E, Serruys PW. Left ventricular unloading and concomitant total cardiac output increase by the use of percutaneous Impella Recover LP 2.5 assist device during high-risk coronary intervention. Catheter Cardiovasc Interv. 2005;65(2):263–7. - PubMed
-
- Meyns B, Dens J, Sergeant P, Herijgers P, Daenen W, Flameng W. Initial experiences with the Impella device in patients with cardiogenic shock—Impella support for cardiogenic shock. Thorac Cardiovac Surg. 2003;51(6):312–7. - PubMed
-
- Sauren LD, Accord RE, Hamzeh K, et al. Combined Impella and intra-aortic balloon pump support to improve both ventricular unloading and coronary blood flow for myocardial recovery: an experimental study. Artif Organs. 2007;31(11):839–42. - PubMed
-
- Toggweiler S, Jamshidi P, Erne P. Functional mitral stenosis: a rare complication of the Impella assist device. Eur J Echocardiogr. 2008 - PubMed
-
- Dixon SR, Henriques JP, Mauri L, et al. A prospective feasibility trial investigating the use of the Impella 2.5 system in patients undergoing high-risk percutaneous coronary intervention (The PROTECT I Trial): initial US experience. JACC Cardiovasc Interv. 2009;2(2):91–6. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous