Structure and dynamics of cationic membrane peptides and proteins: insights from solid-state NMR
- PMID: 21344534
- PMCID: PMC3081543
- DOI: 10.1002/pro.600
Structure and dynamics of cationic membrane peptides and proteins: insights from solid-state NMR
Abstract
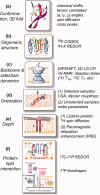
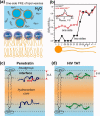
Many membrane peptides and protein domains contain functionally important cationic Arg and Lys residues, whose insertion into the hydrophobic interior of the lipid bilayer encounters significant energy barriers. To understand how these cationic molecules overcome the free energy barrier to insert into the lipid membrane, we have used solid-state NMR spectroscopy to determine the membrane-bound topology of these peptides. A versatile array of solid-state NMR experiments now readily yields the conformation, dynamics, orientation, depth of insertion, and site-specific protein-lipid interactions of these molecules. We summarize key findings of several Arg-rich membrane peptides, including β-sheet antimicrobial peptides, unstructured cell-penetrating peptides, and the voltage-sensing helix of voltage-gated potassium channels. Our results indicate the central role of guanidinium-phosphate and guanidinium-water interactions in dictating the structural topology of these cationic molecules in the lipid membrane, which in turn account for the mechanisms of this functionally diverse class of membrane peptides.
Copyright © 2011 The Protein Society.
Figures



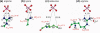
References
-
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. - PubMed
-
- Fischer R, Fotin-Mleczek M, Hufnagel H, Brock R. Break on through to the other side-biophysics and cell biology shed light on cell-penetrating peptides. ChemBioChem. 2005;6:2126–2142. - PubMed
-
- Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R. X-ray structure of a voltage-dependent K+ channel. Nature. 2003;423:33–41. - PubMed
-
- Hessa T, Kim H, Bihlmaier K, Lundin C, Boekel J, Andersson H, Nilsson I, White SH, von Heijne G. Recognition of transmembrane helices by the endoplasmic reticulum translocon. Nature. 2005;433:377–381. - PubMed
-
- Popot JL, Engelman DM. Membrane protein folding and oligomerization: the two-stage model. Biochemistry. 1990;29:4031–4037. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

