Kinetically controlled cellular interactions of polymer-polymer and polymer-liposome nanohybrid systems
- PMID: 21344902
- PMCID: PMC3059376
- DOI: 10.1021/bc100484t
Kinetically controlled cellular interactions of polymer-polymer and polymer-liposome nanohybrid systems
Abstract
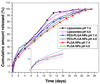
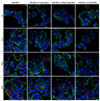
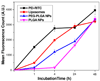
Although bioactive polymers such as cationic polymers have demonstrated potential as drug carriers and nonviral gene delivery vectors, high toxicity and uncontrolled, instantaneous cellular interactions of those vectors have hindered the successful implementation In Vivo. Fine control over the cellular interactions of a potential drug/gene delivery vector would be thus desirable. Herein, we have designed nanohybrid systems (100-150 nm in diameter) that combine the polycations with protective outer layers consisting of biodegradable polymeric nanoparticles (NPs) or liposomes. A commonly used polycation polyethylenimine (PEI) was employed after conjugation with rhodamine (RITC). The PEI-RITC conjugates were then encapsulated into (i) polymeric NPs made of either poly(lactide-co-glycolide) (PLGA) or poly(ethylene glycol)-b-poly(lactide-co-glycolide) (PEG-PLGA); or (ii) PEGylated liposomes, resulting in three nanohybrid systems. Through the nanohybridization, both cellular uptake and cytotoxicity of the nanohybrids were kinetically controlled. The cytotoxicity assay using MCF-7 cells revealed that liposome-based nanohybrids exhibited the least toxicity, followed by PEG-PLGA- and PLGA-based NPs after 24 h incubation. The different kinetics of cellular uptake was also observed, the liposome-based systems being the fastest and PLGA-based systems being the slowest. The results present a potential delivery platform with enhanced control over its biological interaction kinetics and passive targeting capability through size control.
Figures



Similar articles
-
In vitro evaluation of dendrimer-polymer hybrid nanoparticles on their controlled cellular targeting kinetics.Mol Pharm. 2013 Jun 3;10(6):2157-66. doi: 10.1021/mp300560n. Epub 2012 Dec 31. Mol Pharm. 2013. PMID: 23234605 Free PMC article.
-
Arginine-Modified Polymers Facilitate Poly (Lactide-Co-Glycolide)-Based Nanoparticle Gene Delivery to Primary Human Astrocytes.Int J Nanomedicine. 2020 May 22;15:3639-3647. doi: 10.2147/IJN.S250865. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32547019 Free PMC article.
-
Characterization of rhodamine loaded PEG-g-PLA nanoparticles (NPs): effect of poly(ethylene glycol) grafting density.Int J Pharm. 2011 Jun 15;411(1-2):178-87. doi: 10.1016/j.ijpharm.2011.02.039. Epub 2011 Mar 31. Int J Pharm. 2011. PMID: 21458551
-
On the synthesis of mucus permeating nanocarriers.Eur J Pharm Biopharm. 2015 Nov;97(Pt A):239-49. doi: 10.1016/j.ejpb.2015.01.021. Epub 2015 Feb 3. Eur J Pharm Biopharm. 2015. PMID: 25661586
-
Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review.Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):427-43. doi: 10.1016/j.ejpb.2013.07.002. Epub 2013 Jul 17. Eur J Pharm Biopharm. 2013. PMID: 23872180 Review.
Cited by
-
In vitro evaluation of dendrimer-polymer hybrid nanoparticles on their controlled cellular targeting kinetics.Mol Pharm. 2013 Jun 3;10(6):2157-66. doi: 10.1021/mp300560n. Epub 2012 Dec 31. Mol Pharm. 2013. PMID: 23234605 Free PMC article.
-
Tweaking dendrimers and dendritic nanoparticles for controlled nano-bio interactions: potential nanocarriers for improved cancer targeting.J Drug Target. 2015;23(7-8):642-50. doi: 10.3109/1061186X.2015.1052077. J Drug Target. 2015. PMID: 26453160 Free PMC article. Review.
-
Developments in Treatment Methodologies Using Dendrimers for Infectious Diseases.Molecules. 2021 May 31;26(11):3304. doi: 10.3390/molecules26113304. Molecules. 2021. PMID: 34072765 Free PMC article. Review.
-
Effect of size, surface charge, and hydrophobicity of poly(amidoamine) dendrimers on their skin penetration.Biomacromolecules. 2012 Jul 9;13(7):2154-62. doi: 10.1021/bm300545b. Epub 2012 Jun 5. Biomacromolecules. 2012. PMID: 22621160 Free PMC article.
-
PLGA/liposome hybrid nanoparticles for short-chain ceramide delivery.Pharm Res. 2014 Mar;31(3):684-93. doi: 10.1007/s11095-013-1190-5. Epub 2013 Sep 25. Pharm Res. 2014. PMID: 24065591 Free PMC article.
References
-
- Nori A, Kopecek J. Intracellular targeting of polymer-bound drugs for cancer chemotherapy. Adv. Drug Deliv. Rev. 2005;57:609–636. - PubMed
-
- Patri AK, Majoros IJ, Baker JR. Dendritic polymer macromolecular carriers for drug delivery. Curr. Opin. Chem. Biol. 2002;6:466–471. - PubMed
-
- Verma IM, Somia N. Gene therapy - promises, problems and prospects. Nature. 1997;389:239–242. - PubMed
-
- Gebhart CL, Kabanov AV. Evaluation of polyplexes as gene transfer agents. J. Control. Release. 2001;73:401–416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

