Perilipin deficiency and autosomal dominant partial lipodystrophy
- PMID: 21345103
- PMCID: PMC3773916
- DOI: 10.1056/NEJMoa1007487
Perilipin deficiency and autosomal dominant partial lipodystrophy
Abstract
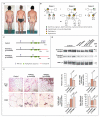
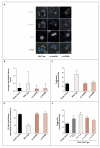
Perilipin is the most abundant adipocyte-specific protein that coats lipid droplets, and it is required for optimal lipid incorporation and release from the droplet. We identified two heterozygous frameshift mutations in the perilipin gene (PLIN1) in three families with partial lipodystrophy, severe dyslipidemia, and insulin-resistant diabetes. Subcutaneous fat from the patients was characterized by smaller-than-normal adipocytes, macrophage infiltration, and fibrosis. In contrast to wild-type perilipin, mutant forms of the protein failed to increase triglyceride accumulation when expressed heterologously in preadipocytes. These findings define a novel dominant form of inherited lipodystrophy and highlight the serious metabolic consequences of a primary defect in the formation of lipid droplets in adipose tissue.
Figures
Similar articles
-
Clinical and molecular characterization of a novel PLIN1 frameshift mutation identified in patients with familial partial lipodystrophy.Diabetes. 2015 Jan;64(1):299-310. doi: 10.2337/db14-0104. Epub 2014 Aug 11. Diabetes. 2015. PMID: 25114292 Free PMC article.
-
Diagnostic Challenge in PLIN1-Associated Familial Partial Lipodystrophy.J Clin Endocrinol Metab. 2019 Dec 1;104(12):6025-6032. doi: 10.1210/jc.2019-00849. J Clin Endocrinol Metab. 2019. PMID: 31504636 Free PMC article.
-
Hypertriglyceridemia Results From an Impaired Catabolism of Triglyceride-Rich Lipoproteins in PLIN1-Related Lipodystrophy.Arterioscler Thromb Vasc Biol. 2024 Aug;44(8):1873-1883. doi: 10.1161/ATVBAHA.124.320774. Epub 2024 Jun 20. Arterioscler Thromb Vasc Biol. 2024. PMID: 38899472
-
Human congenital perilipin deficiency and insulin resistance.Endocr Dev. 2013;24:150-5. doi: 10.1159/000342511. Epub 2013 Feb 1. Endocr Dev. 2013. PMID: 23392103 Review.
-
Perilipin 1: a systematic review on its functions on lipid metabolism and atherosclerosis in mice and humans.Cardiovasc Res. 2024 Mar 14;120(3):237-248. doi: 10.1093/cvr/cvae005. Cardiovasc Res. 2024. PMID: 38214891
Cited by
-
Lipodystrophies: adipose tissue disorders with severe metabolic implications.J Physiol Biochem. 2015 Sep;71(3):471-8. doi: 10.1007/s13105-015-0404-1. Epub 2015 Apr 2. J Physiol Biochem. 2015. PMID: 25833179 Review.
-
Bone imaging findings in genetic and acquired lipodystrophic syndromes: an imaging study of 24 cases.Skeletal Radiol. 2016 Nov;45(11):1495-506. doi: 10.1007/s00256-016-2457-9. Epub 2016 Sep 8. Skeletal Radiol. 2016. PMID: 27631079
-
Adipose tissue: between the extremes.EMBO J. 2017 Jul 14;36(14):1999-2017. doi: 10.15252/embj.201696206. Epub 2017 Jun 16. EMBO J. 2017. PMID: 28623240 Free PMC article. Review.
-
Integrative Analysis of the Predictive Value of Perilipin Family on Clinical Significance, Prognosis and Immunotherapy of Glioma.Biomedicines. 2023 Mar 24;11(4):1009. doi: 10.3390/biomedicines11041009. Biomedicines. 2023. PMID: 37189627 Free PMC article.
-
Null mutation in hormone-sensitive lipase gene and risk of type 2 diabetes.N Engl J Med. 2014 Jun 12;370(24):2307-2315. doi: 10.1056/NEJMoa1315496. Epub 2014 May 21. N Engl J Med. 2014. PMID: 24848981 Free PMC article.
References
-
- Frayn KN. Adipose tissue as a buffer for daily lipid flux. Diabetologia. 2002;45:1201–10. - PubMed
-
- Boutet E, El Mourabit H, Prot M, et al. Seipin deficiency alters fatty acid Delta9 desaturation and lipid droplet formation in Berardinelli-Seip congenital lipodystrophy. Biochimie. 2009;91:796–803. - PubMed
-
- Kim CA, Delépine M, Boutet E, et al. Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. J Clin Endocrinol Metab. 2008;93:1129–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
