Reducing LPS content in cockroach allergens increases pulmonary cytokine production without increasing inflammation: a randomized laboratory study
- PMID: 21345191
- PMCID: PMC3050874
- DOI: 10.1186/1471-2466-11-12
Reducing LPS content in cockroach allergens increases pulmonary cytokine production without increasing inflammation: a randomized laboratory study
Abstract
Background: Endotoxins are ubiquitously present in the environment and constitute a significant component of ambient air. These substances have been shown to modulate the allergic response, however a consensus has yet to be reached whether they attenuate or exacerbate asthmatic responses. The current investigation examined whether reducing the concentration of lipopolysaccharide (LPS) in a house dust extract (HDE) containing high concentrations of both cockroach allergens 1 and LPS would attenuate asthma-like pulmonary inflammation.
Methods: Mice were sensitized with CRA and challenged with the intact HDE, containing 182 ng of LPS, or an LPS-reduced HDE containing 3 ng LPS, but an equivalent amount of CRA. Multiple parameters of asthma-like pulmonary inflammation were measured.
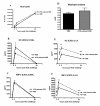
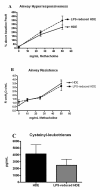
Results: Compared to HDE challenged mice, the LPS-reduced HDE challenged mice had significantly reduced TNFα levels in the bronchoalveolar lavage fluid. Plasma levels of IgE and IgG1 were significantly reduced, however no change in CRA-specific IgE was detected. In HDE mice, plasma IgG2a levels were similar to naïve mice, while LPS-reduced HDE mice had significantly greater concentrations. Reduced levels of LPS in the HDE did not decrease eosinophil or neutrophil recruitment into the alveolar space. Equivalent inflammatory cell recruitment occurred despite having generally higher pulmonary concentrations of eotaxins and CXC chemokines in the LPS-reduced HDE group. LPS-reduced HDE challenge induced significantly higher concentrations of IFNγ, and IL-5 and IL-13 in the BAL fluid, but did not decrease airways hyperresponsiveness or airway resistance to methacholine challenge.
Conclusion: These data show that reduction of LPS levels in the HDE does not significantly protect against the severity of asthma-like pulmonary inflammation.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

