Are voltage-gated sodium channels on the dorsal root ganglion involved in the development of neuropathic pain?
- PMID: 21345196
- PMCID: PMC3052185
- DOI: 10.1186/1744-8069-7-16
Are voltage-gated sodium channels on the dorsal root ganglion involved in the development of neuropathic pain?
Abstract
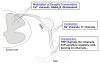
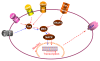
Neuropathic pain is a common clinical condition. Current treatments are often inadequate, ineffective, or produce potentially severe adverse effects. Understanding the mechanisms that underlie the development and maintenance of neuropathic pain will be helpful in identifying new therapeutic targets and developing effective strategies for the prevention and/or treatment of this disorder. The genesis of neuropathic pain is reliant, at least in part, on abnormal spontaneous activity within sensory neurons. Therefore, voltage-gated sodium channels, which are essential for the generation and conduction of action potentials, are potential targets for treating neuropathic pain. However, preclinical studies have shown unexpected results because most pain-associated voltage-gated channels in the dorsal root ganglion are down-regulated after peripheral nerve injury. The role of dorsal root ganglion voltage-gated channels in neuropathic pain is still unclear. In this report, we describe the expression and distribution of voltage-gated sodium channels in the dorsal root ganglion. We also review evidence regarding changes in their expression under neuropathic pain conditions and their roles in behavioral responses in a variety of neuropathic pain models. We finally discuss their potential involvement in neuropathic pain.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

