Alpha-1 antitrypsin protein and gene therapies decrease autoimmunity and delay arthritis development in mouse model
- PMID: 21345239
- PMCID: PMC3050720
- DOI: 10.1186/1479-5876-9-21
Alpha-1 antitrypsin protein and gene therapies decrease autoimmunity and delay arthritis development in mouse model
Abstract
Background: Alpha-1 antitrypsin (AAT) is a multi-functional protein that has anti-inflammatory and tissue protective properties. We previously reported that human AAT (hAAT) gene therapy prevented autoimmune diabetes in non-obese diabetic (NOD) mice and suppressed arthritis development in combination with doxycycline in mice. In the present study we investigated the feasibility of hAAT monotherapy for the treatment of chronic arthritis in collagen-induced arthritis (CIA), a mouse model of rheumatoid arthritis (RA).
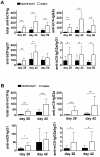
Methods: DBA/1 mice were immunized with bovine type II collagen (bCII) to induce arthritis. These mice were pretreated either with hAAT protein or with recombinant adeno-associated virus vector expressing hAAT (rAAV-hAAT). Control groups received saline injections. Arthritis development was evaluated by prevalence of arthritis and arthritic index. Serum levels of B-cell activating factor of the TNF-α family (BAFF), antibodies against both bovine (bCII) and mouse collagen II (mCII) were tested by ELISA.
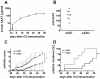
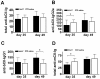
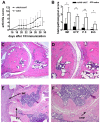
Results: Human AAT protein therapy as well as recombinant adeno-associated virus (rAAV8)-mediated hAAT gene therapy significantly delayed onset and ameliorated disease development of arthritis in CIA mouse model. Importantly, hAAT therapies significantly reduced serum levels of BAFF and autoantibodies against bCII and mCII, suggesting that the effects are mediated via B-cells, at least partially.
Conclusion: These results present a new drug for arthritis therapy. Human AAT protein and gene therapies are able to ameliorate and delay arthritis development and reduce autoimmunity, indicating promising potential of these therapies as a new treatment strategy for RA.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

