A tryptophan-rich motif in the human parainfluenza virus type 2 V protein is critical for the blockade of toll-like receptor 7 (TLR7)- and TLR9-dependent signaling
- PMID: 21345944
- PMCID: PMC3126260
- DOI: 10.1128/JVI.02012-10
A tryptophan-rich motif in the human parainfluenza virus type 2 V protein is critical for the blockade of toll-like receptor 7 (TLR7)- and TLR9-dependent signaling
Abstract
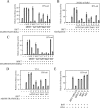
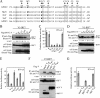
Plasmacytoid dendritic cells (pDCs) do not produce alpha interferon (IFN-α) unless viruses cause a systemic infection or overcome the first-line defense provided by conventional DCs and macrophages. We show here that even paramyxoviruses, whose infections are restricted to the respiratory tract, have a V protein able to prevent Toll-like receptor 7 (TLR7)- and TLR9-dependent IFN-α induction specific to pDCs. Mutational analysis of human parainfluenza virus type 2 demonstrates that the second Trp residue of the Trp-rich motif (Trp-X(3)-Trp-X(9)-Trp) in the C-terminal domain unique to V, a determinant for IRF7 binding, is critical for the blockade of TLR7/9-dependent signaling.
Figures


References
-
- Cao W., Liu Y. J. 2007. Innate immune functions of plasmacytoid dendritic cells. Curr. Opin. Immunol. 19:24–30 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

