Cytomegalovirus UL103 controls virion and dense body egress
- PMID: 21345947
- PMCID: PMC3126192
- DOI: 10.1128/JVI.01682-10
Cytomegalovirus UL103 controls virion and dense body egress
Abstract
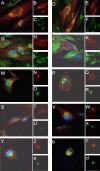
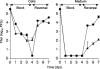
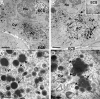
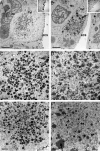
Human cytomegalovirus UL103 encodes a tegument protein that is conserved across herpesvirus subgroups. Mutant viruses lacking this gene product exhibit dramatically reduced accumulation of cell-free virus progeny and poor cell-to-cell spread. Given that viral proteins and viral DNA accumulate with normal kinetics in cells infected with mutant virus, UL103 appears to function during the late phase of replication, playing a critical role in egress of capsidless dense bodies and virions. Few dense bodies were observed in the extracellular space in mutant virus-infected cells in the presence or absence of the DNA encapsidation inhibitor 2-bromo-5,6-dichloro-1-(β-d-ribofuranosyl)benzimidazole. Upon reversal of encapsidation inhibition, UL103 had a striking impact on accumulation of cell-free virus, but not on accumulation of cell-associated virus. Thus, UL103 plays a novel and important role during maturation, regulating virus particle and dense body egress from infected cells.
Figures

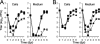


References
-
- Bartz S. R., Vodicka M. A. 1997. Production of high-titer human immunodeficiency virus type 1 pseudotyped with vesicular stomatitis virus glycoprotein. Methods 12:337–342 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

