Induction of immune tolerance in asthmatic mice by vaccination with DNA encoding an allergen-cytotoxic T lymphocyte-associated antigen 4 combination
- PMID: 21346053
- PMCID: PMC3122522
- DOI: 10.1128/CVI.00434-10
Induction of immune tolerance in asthmatic mice by vaccination with DNA encoding an allergen-cytotoxic T lymphocyte-associated antigen 4 combination
Abstract
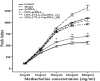
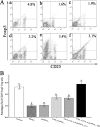
Allergen-specific immunotherapy is a potential treatment for allergic diseases. We constructed an allergen-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4)-encoding DNA vaccine, administered it directly to antigen-presenting cells (APCs), and investigated its ability and mechanisms to ameliorate allergic airway inflammation in an asthmatic mouse model. An allergen-CTLA-4 DNA plasmid (OVA-CTLA-4-pcDNA₃.₁) encoding an ovalbumin (OVA) and the mouse CTLA-4 extracellular domain was constructed and transfected into COS-7 cells to obtain the fusion protein OVA-CTLA-4, which was able to bind the B7 ligand on dendritic cells (DCs), and induced CD25⁺ Foxp3⁺ regulatory T (Treg) cells by the coculture of naive CD4⁺ T cells with DCs in vitro. In an animal study, BALB/c mice were sensitized and challenged with OVA to establish the asthmatic model. Vaccination with a high dose of OVA-CTLA-4-pcDNA₃.₁ significantly decreased interleukin-4 (IL-4) and IL-5 levels and eosinophil counts and prevented OVA-induced reduction of the gamma interferon level in the bronchoalveolar lavage fluid. In addition, these mice suffered less severe airway inflammation and had lower levels of OVA-specific IgE and IgG1 titers in serum. Also, high-dose OVA-CTLA-4-pcDNA₃.₁ vaccination inhibited the development of airway hyperreactivity and prevented OVA-induced reduction of the percentages of Foxp3⁺ Treg cells in the spleen. Our results indicate that a high dose of allergen-CTLA-4-encoding DNA vaccine was more effective in preventing an allergen-induced Th2-skewed immune response through the induction of Treg cells and may be a new alternative therapy for asthma.
Figures




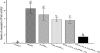
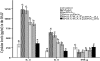
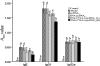
References
-
- Abe M., et al. 2008. Foxp3 expression on normal and leukemic CD4+ CD25+ T cells implicated in human T-cell leukemia virus type-1 is inconsistent with Treg cells. Eur. J. Haematol. 81:209–217 - PubMed
-
- Ahrens B., et al. 2009. BCG priming of dendritic cells enhances T regulatory and Th1 function and suppresses allergen-induced Th2 function in vitro and in vivo. Int. Arch. Allergy Immunol. 150:210–220 - PubMed
-
- Akdis M., Akdis C. A. 2007. Mechanisms of allergen-specific immunotherapy. J. Allergy Clin. Immunol. 119:780–791 - PubMed
-
- Cosmi L., et al. 2004. Th2 cells are less susceptible than Th1 cells to the suppressive activity of CD25+ regulatory thymocytes because of their responsiveness to different cytokines. Blood 103:3117–3121 - PubMed
-
- Cusi M. G., et al. 2004. Efficient delivery of DNA to dendritic cells mediated by influenza virosomes. Vaccine 22:735–739 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

