Shmt1 and de novo thymidylate biosynthesis underlie folate-responsive neural tube defects in mice
- PMID: 21346092
- PMCID: PMC3057548
- DOI: 10.3945/ajcn.110.002766
Shmt1 and de novo thymidylate biosynthesis underlie folate-responsive neural tube defects in mice
Abstract
Background: Folic acid supplementation prevents the occurrence and recurrence of neural tube defects (NTDs), but the causal metabolic pathways underlying folic acid-responsive NTDs have not been established. Serine hydroxymethyltransferase (SHMT1) partitions folate-derived one-carbon units to thymidylate biosynthesis at the expense of cellular methylation, and therefore SHMT1-deficient mice are a model to investigate the metabolic origin of folate-associated pathologies.
Objectives: We examined whether genetic disruption of the Shmt1 gene in mice induces NTDs in response to maternal folate and choline deficiency and whether a corresponding disruption in de novo thymidylate biosynthesis underlies NTD pathogenesis.
Design: Shmt1 wild-type, Shmt1(+/-), and Shmt1(-/-) mice fed either folate- and choline-sufficient or folate- and choline-deficient diets were bred, and litters were examined for the presence of NTDs. Biomarkers of impaired folate metabolism were measured in the dams. In addition, the effect of Shmt1 disruption on NTD incidence was investigated in Pax3(Sp) mice, an established folate-responsive NTD mouse model.
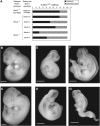
Results: Shmt1(+/-) and Shmt1(-/-) embryos exhibited exencephaly in response to maternal folate and choline deficiency. Shmt1 disruption on the Pax3(Sp) background exacerbated NTD frequency and severity. Pax3 disruption impaired de novo thymidylate and purine biosynthesis and altered amounts of SHMT1 and thymidylate synthase protein.
Conclusions: SHMT1 is the only folate-metabolizing enzyme that has been shown to affect neural tube closure in mice by directly inhibiting folate metabolism. These results provide evidence that disruption of Shmt1 expression causes NTDs by impairing thymidylate biosynthesis and shows that changes in the expression of genes that encode folate-dependent enzymes may be key determinates of NTD risk.
Figures


Comment in
-
Folate-responsive birth defects: of mice and women.Am J Clin Nutr. 2012 Jan;95(1):1-2. doi: 10.3945/ajcn.111.029595. Epub 2011 Dec 7. Am J Clin Nutr. 2012. PMID: 22158725 No abstract available.
References
-
- International Clearinghouse for Birth Defects Monitoring Systems. World atlas of birth defects. 2 ed. Geneva, Switzerland: World Health Organization, 2003
-
- Kirke PN, Molloy AM, Daly LE, Burke H, Weir DG, Scott JM. Maternal plasma folate and vitamin B12 are independent risk factors for neural tube defects. Q J Med 1993;86:703–8 - PubMed
-
- Relton CL, Wilding CS, Laffling AJ, et al. Low erythrocyte folate status and polymorphic variation in folate-related genes are associated with risk of neural tube defect pregnancy. Mol Genet Metab 2004;81:273–81 - PubMed
-
- Christensen B, Arbour L, Tran P, et al. Genetic polymorphisms in methylenetetrahydrofolate reductase and methionine synthase, folate levels in red blood cells, and risk of neural tube defects. Am J Med Genet 1999;84:151–7 - PubMed
-
- Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med 1992;327:1832–5 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

