Redistribution of the tight junction protein ZO-1 during physiological shedding of mouse intestinal epithelial cells
- PMID: 21346149
- PMCID: PMC3118625
- DOI: 10.1152/ajpcell.00270.2010
Redistribution of the tight junction protein ZO-1 during physiological shedding of mouse intestinal epithelial cells
Abstract
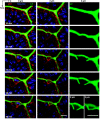
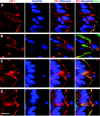
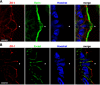
We questioned how tight junctions contribute to intestinal barrier function during the cell shedding that is part of physiological cell renewal. Intravital confocal microscopy studied the jejunal villus epithelium of mice expressing a fluorescent zonula occludens 1 (ZO-1) fusion protein. Vital staining also visualized the cell nucleus (Hoechst staining) or local permeability to luminal constituents (Lucifer Yellow; LY). In a cell fated to be shed, ZO-1 redistributes from the tight junction toward the apical and then basolateral cell region. ZO-1 rearrangement occurs 15 ± 6 min (n = 28) before movement of the cell nucleus from the epithelial layer. During cell extrusion, permeation of luminal LY extends along the lateral intercellular spaces of the shedding cell only as far as the location of ZO-1. Within 3 min after detachment from the epithelial layer, nuclear chromatin condenses. After cell loss, a residual patch of ZO-1 remains in the space previously occupied by the departed cell, and the size of the patch shrinks to 14 ± 2% (n = 15) of the original cell space over 20 min. The duration of cell shedding measured by nucleus movement (14 ± 1 min) is much less than the total duration of ZO-1 redistribution at the same sites (45 ± 2 min). In about 15% of cell shedding cases, neighboring epithelial cells also undergo extrusion with a delay of 5-10 min. With the use of normal mice, ZO-1 immunofluorescent staining of fixed tissue confirmed ZO-1 redistribution and the presence of ZO-1 patches beneath shedding cells. Immunostaining also showed that redistribution of ZO-1 occurred without corresponding mixing of apical and basolateral membrane domains as marked by ezrin or E-cadherin. ZO-1 redistribution is the earliest cellular event yet identified as a herald of physiological cell shedding, and redistribution of tight junction function along the lateral plasma membrane sustains epithelial barrier during cell shedding.
Figures




References
-
- Baron DA, Miller DH. Extrusion of colonic epithelial cells in vitro. J Electron Microsc Tech 16: 15–24, 1990 - PubMed
-
- Bement WM, Mandato CA, Kirsch MN. Wound-induced assembly and closure of an actomyosin purse string in Xenopus oocytes. Curr Biol 9: 579–587, 1999 - PubMed
-
- Bullen TF, Forrest S, Campbell F, Dodson AR, Hershman MJ, Pritchard DM, Turner JR, Montrose MH, Watson AJ. Characterization of epithelial cell shedding from human small intestine. Lab Invest 86: 1052–1063, 2006 - PubMed
-
- Chen L, Park SM, Turner JR, Peter ME. Cell death in the colonic epithelium during inflammatory bowel diseases: CD95/Fas and beyond. Inflamm Bowel Dis 16: 1071–1076 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

