Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome
- PMID: 21346177
- PMCID: PMC3064099
- DOI: 10.2337/db10-0916
Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome
Abstract
Objective: Hyperuricemia is strongly associated with obesity and metabolic syndrome and can predict visceral obesity and insulin resistance. Previously, we showed that soluble uric acid directly stimulated the redox-dependent proinflammatory signaling in adipocytes. In this study we demonstrate the role of hyperuricemia in the production of key adipokines.
Research design and methods: We used mouse 3T3-L1 adipocytes, human primary adipocytes, and a mouse model of metabolic syndrome and hyperuricemia.
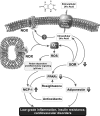
Results: Uric acid induced in vitro an increase in the production (mRNA and secreted protein) of monocyte chemotactic protein-1 (MCP-1), an adipokine playing an essential role in inducing the proinflammatory state in adipocytes in obesity. In addition, uric acid caused a decrease in the production of adiponectin, an adipocyte-specific insulin sensitizer and anti-inflammatory agent. Uric acid-induced increase in MCP-1 production was blocked by scavenging superoxide or by inhibiting NADPH oxidase and by stimulating peroxisome-proliferator-activated receptor-γ with rosiglitazone. Downregulation of the adiponectin production was prevented by rosiglitazone but not by antioxidants. In obese mice with metabolic syndrome, we observed hyperuricemia. Lowering uric acid in these mice by inhibiting xanthine oxidoreductase with allopurinol could improve the proinflammatory endocrine imbalance in the adipose tissue by reducing production of MCP-1 and increasing production of adiponectin. In addition, lowering uric acid in obese mice decreased macrophage infiltration in the adipose tissue and reduced insulin resistance.
Conclusions: Hyperuricemia might be partially responsible for the proinflammatory endocrine imbalance in the adipose tissue, which is an underlying mechanism of the low-grade inflammation and insulin resistance in subjects with the metabolic syndrome.
Figures







References
-
- Johnson RJ, Segal MS, Srinivas T, et al. Essential hypertension, progressive renal disease, and uric acid: a pathogenetic link? J Am Soc Nephrol 2005;16:1909–1919 - PubMed
-
- Matsuura F, Yamashita S, Nakamura T, et al. Effect of visceral fat accumulation on uric acid metabolism in male obese subjects: visceral fat obesity is linked more closely to overproduction of uric acid than subcutaneous fat obesity. Metabolism 1998;47:929–933 - PubMed
-
- Hikita M, Ohno I, Mori Y, Ichida K, Yokose T, Hosoya T. Relationship between hyperuricemia and body fat distribution. Intern Med 2007;46:1353–1358 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

