HIV-1 requires Arf6-mediated membrane dynamics to efficiently enter and infect T lymphocytes
- PMID: 21346189
- PMCID: PMC3078069
- DOI: 10.1091/mbc.E10-08-0722
HIV-1 requires Arf6-mediated membrane dynamics to efficiently enter and infect T lymphocytes
Abstract
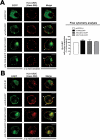
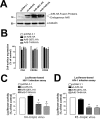
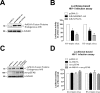
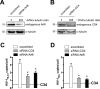
As the initial barrier to viral entry, the plasma membrane along with the membrane trafficking machinery and cytoskeleton are of fundamental importance in the viral cycle. However, little is known about the contribution of plasma membrane dynamics during early human immunodeficiency virus type 1 (HIV-1) infection. Considering that ADP ribosylation factor 6 (Arf6) regulates cellular invasion via several microorganisms by coordinating membrane trafficking, our aim was to study the function of Arf6-mediated membrane dynamics on HIV-1 entry and infection of T lymphocytes. We observed that an alteration of the Arf6-guanosine 5'-diphosphate/guanosine 5'-triphosphate (GTP/GDP) cycle, by GDP-bound or GTP-bound inactive mutants or by specific Arf6 silencing, inhibited HIV-1 envelope-induced membrane fusion, entry, and infection of T lymphocytes and permissive cells, regardless of viral tropism. Furthermore, cell-to-cell HIV-1 transmission of primary human CD4(+) T lymphocytes was inhibited by Arf6 knockdown. Total internal reflection fluorescence microscopy showed that Arf6 mutants provoked the accumulation of phosphatidylinositol-(4,5)-biphosphate-associated structures on the plasma membrane of permissive cells, without affecting CD4-viral attachment but impeding CD4-dependent HIV-1 entry. Arf6 silencing or its mutants did not affect fusion, entry, and infection of vesicular stomatitis virus G-pseudotyped viruses or ligand-induced CXCR4 or CCR5 endocytosis, both clathrin-dependent processes. Therefore we propose that efficient early HIV-1 infection of CD4(+) T lymphocytes requires Arf6-coordinated plasma membrane dynamics that promote viral fusion and entry.
Figures





Similar articles
-
A Membrane-Anchored Short-Peptide Fusion Inhibitor Fully Protects Target Cells from Infections of Human Immunodeficiency Virus Type 1 (HIV-1), HIV-2, and Simian Immunodeficiency Virus.J Virol. 2019 Oct 29;93(22):e01177-19. doi: 10.1128/JVI.01177-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31462566 Free PMC article.
-
Role of HIV-1 subtype C envelope V3 to V5 regions in viral entry, coreceptor utilization and replication efficiency in primary T-lymphocytes and monocyte-derived macrophages.Virol J. 2007 Nov 24;4:126. doi: 10.1186/1743-422X-4-126. Virol J. 2007. PMID: 18036244 Free PMC article.
-
A recombinant vesicular stomatitis virus encoding CCR5-tropic HIV-1 receptors targets HIV-1-infected cells and controls HIV-1 infection.Microbes Infect. 2017 Apr-May;19(4-5):277-287. doi: 10.1016/j.micinf.2016.12.004. Epub 2016 Dec 24. Microbes Infect. 2017. PMID: 28025070
-
Coreceptors and HIV-1 pathogenesis.Curr HIV/AIDS Rep. 2011 Mar;8(1):45-53. doi: 10.1007/s11904-010-0069-x. Curr HIV/AIDS Rep. 2011. PMID: 21188555 Review.
-
Asymmetric HIV-1 co-receptor use and replication in CD4(+) T lymphocytes.J Transl Med. 2011 Jan 27;9 Suppl 1(Suppl 1):S8. doi: 10.1186/1479-5876-9-S1-S8. J Transl Med. 2011. PMID: 21284907 Free PMC article. Review.
Cited by
-
ceRNA Network Analysis Reveals Potential Key miRNAs and Target Genes in COVID-19-Related Chronic Obstructive Pulmonary Disease.Appl Biochem Biotechnol. 2024 Jul;196(7):4303-4316. doi: 10.1007/s12010-023-04773-7. Epub 2023 Nov 10. Appl Biochem Biotechnol. 2024. PMID: 37947947
-
ATF4 Signaling in HIV-1 Infection: Viral Subversion of a Stress Response Transcription Factor.Biology (Basel). 2024 Feb 26;13(3):146. doi: 10.3390/biology13030146. Biology (Basel). 2024. PMID: 38534416 Free PMC article. Review.
-
HIV entry and envelope glycoprotein-mediated fusion.J Biol Chem. 2012 Nov 30;287(49):40841-9. doi: 10.1074/jbc.R112.406272. Epub 2012 Oct 5. J Biol Chem. 2012. PMID: 23043104 Free PMC article. Review.
-
The productive entry pathway of HIV-1 in macrophages is dependent on endocytosis through lipid rafts containing CD4.PLoS One. 2014 Jan 22;9(1):e86071. doi: 10.1371/journal.pone.0086071. eCollection 2014. PLoS One. 2014. PMID: 24465876 Free PMC article.
-
HIV-1 Hijacking of Host ATPases and GTPases That Control Protein Trafficking.Front Cell Dev Biol. 2021 Jul 8;9:622610. doi: 10.3389/fcell.2021.622610. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34307340 Free PMC article. Review.
References
-
- Aikawa Y, Martin TF. ADP-ribosylation factor 6 regulation of phosphatidylinositol-4,5-bisphosphate synthesis, endocytosis, and exocytosis. Methods Enzymol. 2005;404:422–431. - PubMed
-
- Albertson R, Riggs B, Sullivan W. Membrane traffic: a driving force in cytokinesis. Trends Cell Biol. 2005;15:92–101. - PubMed
-
- Barrero-Villar M, Barroso-Gonzalez J, Cabrero JR, Gordon-Alonso M, Alvarez-Losada S, Munoz-Fernandez MA, Sanchez-Madrid F, Valenzuela-Fernandez A. PI4P5-kinase Iα is required for efficient HIV-1 entry and infection of T-cells. J Immunol. 2008;181:6882–6888. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

